Current Understanding of the Role of Autophagy in the Treatment of Myeloid Leukemia
- PMID: 39596291
- PMCID: PMC11594995
- DOI: 10.3390/ijms252212219
Current Understanding of the Role of Autophagy in the Treatment of Myeloid Leukemia
Abstract
The most important issues in acute myeloid leukemia are preventing relapse and treating relapse. Although the remission rate has improved to approximately 80%, the 5-year survival rate is only around 30%. The main reasons for this are the high relapse rate and the limited treatment options. In chronic myeloid leukemia patients, when a deep molecular response is achieved for a certain period of time through tyrosine kinase inhibitor treatment, about half of them will reach treatment-free remission, but relapse is still a problem. Therefore, potential therapeutic targets for myeloid leukemias are eagerly awaited. Autophagy suppresses the development of cancer by maintaining cellular homeostasis; however, it also promotes cancer progression by helping cancer cells survive under various metabolic stresses. In addition, autophagy is promoted or suppressed in cancer cells by various genetic mutations. Therefore, the development of therapies that target autophagy is also being actively researched in the field of leukemia. In this review, studies of the role of autophagy in hematopoiesis, leukemogenesis, and myeloid leukemias are presented, and the impact of autophagy regulation on leukemia treatment and the clinical trials of autophagy-related drugs to date is discussed.
Keywords: acute myeloid leukemia; chronic myeloid leukemia; clinical trial; cyclodextrin; folate receptor; folic acid; hydroxypropyl-β-cyclodextrin; mitophagy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
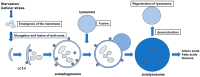

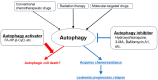
Similar articles
-
Folate-Appended Hydroxypropyl-β-Cyclodextrin Induces Autophagic Cell Death in Acute Myeloid Leukemia Cells.Int J Mol Sci. 2023 Nov 24;24(23):16720. doi: 10.3390/ijms242316720. Int J Mol Sci. 2023. PMID: 38069042 Free PMC article.
-
Role of autophagy in acute myeloid leukemia therapy.Chin J Cancer. 2013 Mar;32(3):130-5. doi: 10.5732/cjc.012.10073. Epub 2012 Aug 2. Chin J Cancer. 2013. PMID: 22854065 Free PMC article. Review.
-
Current Outlook on Autophagy in Human Leukemia: Foe in Cancer Stem Cells and Drug Resistance, Friend in New Therapeutic Interventions.Int J Mol Sci. 2019 Jan 22;20(3):461. doi: 10.3390/ijms20030461. Int J Mol Sci. 2019. PMID: 30678185 Free PMC article. Review.
-
Heme oxygenase-1: A new druggable target in the management of chronic and acute myeloid leukemia.Eur J Med Chem. 2017 Dec 15;142:163-178. doi: 10.1016/j.ejmech.2017.07.031. Epub 2017 Jul 20. Eur J Med Chem. 2017. PMID: 28756878 Review.
-
Roles of the bone marrow niche in hematopoiesis, leukemogenesis, and chemotherapy resistance in acute myeloid leukemia.Hematology. 2018 Dec;23(10):729-739. doi: 10.1080/10245332.2018.1486064. Epub 2018 Jun 14. Hematology. 2018. PMID: 29902132 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

