Assessing the Prognostic Value of Cytoplasmic and Stromal Caveolin-1 in Early Triple-Negative Breast Cancer Undergoing Neoadjuvant Chemotherapy
- PMID: 39596307
- PMCID: PMC11594706
- DOI: 10.3390/ijms252212241
Assessing the Prognostic Value of Cytoplasmic and Stromal Caveolin-1 in Early Triple-Negative Breast Cancer Undergoing Neoadjuvant Chemotherapy
Abstract
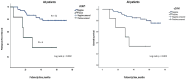
Triple-negative breast cancer (TNBC) is a highly aggressive subtype with limited therapeutic options, leading to higher relapse rates and mortality. Identifying prognostic biomarkers like caveolin-1 (CAV1) is crucial for personalized treatment. CAV1 influences tumor progression and chemotherapy response, particularly through its interaction with the tumor microenvironment (TME) and cancer metabolism. Understanding the prognostic value of CAV1 in different cellular compartments is essential for its clinical application in TNBC. In the methods section CAV1 gene expression in TNBC was evaluated using in silico analysis, followed by the immunohistochemical staining of tumor cytoplasm (cCAV1) and stromal cells (sCAV1) in 58 early-stage TNBC patients. Statistical analyses were performed to correlate CAV1 expression with clinicopathological features and survival. In the results section, in silico analysis revealed higher CAV1 expression in TNBC, correlating with shorter overall survival. In the patient samples, cCAV1 was observed in 10.3% of cases, and was associated with larger tumors, higher grades, and poorer prognoses. sCAV1 was detected in 42% of cases, associated with less proliferative and less aggressive tumors, but did not significantly impact prognoses. In conclusion, cCAV1 expression is a significant prognostic marker in early-stage TNBC, highlighting the importance of assessing CAV1 in different cellular compartments. Further research is needed to explore the mechanisms and clinical implications of cCAV1.
Keywords: CAV1; TME; TNBC; breast cancer prognosis.
Conflict of interest statement
I.T. declares travel expenses from Roche, Novartis and MSD. Educational activities from Novartis, MSD, Lilly, Pfizer and GSK. Congress assistance from Novartis, MSD and Roche. S.R. declares honoraria from Gilead, Novartis, and Roche as invited speaker and from Astra Zeneca, Novartis, and Accord for travel or inscription expenses. E.F declares travel expenses from Pfizer, Lilly, Novartis, and Roche. Speaking fees from Pfizer and advisory board from Novartis. M.B. declares advisory funding from Eisai, AstraZeneca, Pfizer, Novartis, and travel expenses from Novartis and AstraZeneca. B.C. declares being invited as speaker for BMS, Merck, and MSD. Training grants from BMS, Merck, and MSD. Advisory board: BMS, Merck, and MSD. V.Q. declares being invited as speaker for AstraZeneca, Novartis, Pfizer, and Roche. Advisory board for Roche. Educational activities from GSK, Lilly, and Pfizer and travel expenses from Pfizer and Roche. Training grants from Novartis, Lilly, ROCHE, and MSD. A.F.-D. declares being invited as speaker for MSD and Angelini Pharma; and travel expenses from MSD, Lilly, Roche, Merck, and BMS. A.P. declares being invited as speaker for GSK, Eisai, and Lilly, travel expenses and congress assistance from Lilly, Gilead, Dr. Reddys, and Pfizer. A.L. declares being invited as speaker for Eisai, Lilly, and Novartis, and travel expenses from Roche, Gilead, and Novartis. M.R. declares advisory funding from GSK and AstraZeneca, and travel expenses from MSD and AstraZeneca. R.M. declares honoraria received from Merck, Nanobiotics, MSD, Seattle Genetics, AZ, Pfizer, Bayer, Boehringer as advisory role and Merck, MSD and Boehringer as speaker. M.M. declares being invited as speaker for Pfizer, Novartis, and Lilly. Institution research grants from Pfizer and Gilead. Consultant advisory board from Novartis, Lilly, and Menarini. Travel expenses and congress assistance from Gilead, Pfizer, and Roche. E.C., G.V., A.P., M.T., E.M.-B., A.B.-P., L.B., G.S., J.R., P.G., E.B. and A.M.-C. declare no conflicts of interest.
Figures


References
-
- Burstein H.J., Curigliano G., Loibl S., Dubsky P., Gnant M., Poortmans P., Colleoni M., Denkert C., Piccart-Gebhart M., Regan M., et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann. Oncol. 2019;30:1541–1557. - PubMed

