Chemical Landscape of Adipocytes Derived from 3T3-L1 Cells Investigated by Fourier Transform Infrared and Raman Spectroscopies
- PMID: 39596337
- PMCID: PMC11595028
- DOI: 10.3390/ijms252212274
Chemical Landscape of Adipocytes Derived from 3T3-L1 Cells Investigated by Fourier Transform Infrared and Raman Spectroscopies
Abstract
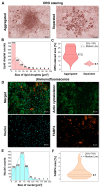
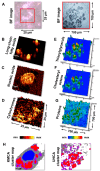
Adipocytes derived from 3T3-L1 cells are a gold standard for analyses of adipogenesis processes and the metabolism of fat cells. A widely used histological and immunohistochemical staining and mass spectrometry lipidomics are mainly aimed for examining lipid droplets (LDs). Visualizing other cellular compartments contributing to the cellular machinery requires additional cell culturing for multiple labeling. Here, we present the localization of the intracellular structure of the 3T3-L1-derived adipocytes utilizing vibrational spectromicroscopy, which simultaneously illustrates the cellular compartments and provides chemical composition without extensive sample preparation and in the naïve state. Both vibrational spectra (FTIR-Fourier transform infrared and RS-Raman scattering spectroscopy) extended the gathered chemical information. We proved that both IR and RS spectra provide distinct chemical information about lipid content and their structure. Despite the expected presence of triacylglycerols and cholesteryl esters in lipid droplets, we also estimated the length and unsaturation degree of the fatty acid acyl chains that were congruent with known MS lipidomics of these cells. In addition, the clustering of spectral images revealed that the direct surroundings around LDs attributed to lipid-associated proteins and a high abundance of mitochondria. Finally, by using quantified markers of biomolecules, we showed that the fixative agents, paraformaldehyde and glutaraldehyde, affected the cellular compartment differently. We concluded that PFA preserves LDs better, while GA fixation is better for cytochromes and unsaturated lipid analysis. The proposed analysis of the spectral images constitutes a complementary tool for investigations into the structural and molecular features of fat cells.
Keywords: FTIR and Raman spectroscopy imaging; adipocytes; cellular compartments; histochemical staining.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Sanjabi B., Dashty M., Özcan B., Akbarkhanzadeh V., Rahimi M., Vinciguerra M., Van Rooij F., Al-Lahham S., Sheedfar F., Van Kooten T.G., et al. Lipid Droplets Hypertrophy: A Crucial Determining Factor in Insulin Regulation by Adipocytes. Sci. Rep. 2015;5:4–6. doi: 10.1038/srep08816. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- Diamond Grant, no. DI2018 018048/Ministry of Science and Higher Education, Poland
- grant "Kościuszko" # 508/2017/DA/Polish Ministry of National Defense
- grant No 5/491340/SPUB/SN/2021/Military Institute of Medicine - National Research Institute
- 35/598515/SPUB/SP/2024/Jagiellonian University in Krakow, the Ministry of Science and Higher Education in Poland
LinkOut - more resources
Full Text Sources

