Mineralocorticoid Receptor and Sleep Quality in Chronic Kidney Disease
- PMID: 39596384
- PMCID: PMC11594958
- DOI: 10.3390/ijms252212320
Mineralocorticoid Receptor and Sleep Quality in Chronic Kidney Disease
Abstract
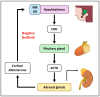
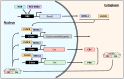
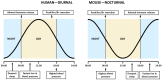
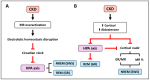
The classical function of the mineralocorticoid receptor (MR) is to maintain electrolytic homeostasis and control extracellular volume and blood pressure. The MR is expressed in the central nervous system (CNS) and is involved in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis as well as sleep physiology, playing a role in the non-rapid eye movement (NREM) phase of sleep. Some patients with psychiatric disorders have very poor sleep quality, and a relationship between MR dysregulation and this disorder has been found in them. In addition, the MR is involved in the regulation of the renal peripheral clock. One of the most common comorbidities observed in patients with chronic kidney disease (CKD) is poor sleep quality. Patients with CKD experience sleep disturbances, including reduced sleep duration, sleep fragmentation, and insomnia. To date, no studies have specifically investigated the relationship between MR activation and CKD-associated sleep disturbances. However, in this review, we analyzed the environment that occurs in CKD and proposed two MR-related mechanisms that may be responsible for these sleep disturbances: the circadian clock disruption and the high levels of MR agonist observed in CKD.
Keywords: chronic kidney disease; circadian clock; mineralocorticoid receptor; sleep quality.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

