Microsomal Prostaglandin E Synthase-1 Controls Colonic Prostaglandin E2 Production and Exerts a Protective Effect on Colitis Induced by Trinitrobenzene Sulfonic Acid in Mice
- PMID: 39596393
- PMCID: PMC11594388
- DOI: 10.3390/ijms252212326
Microsomal Prostaglandin E Synthase-1 Controls Colonic Prostaglandin E2 Production and Exerts a Protective Effect on Colitis Induced by Trinitrobenzene Sulfonic Acid in Mice
Abstract
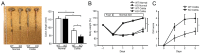
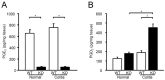
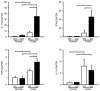
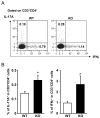
Microsomal prostaglandin E synthase-1 (mPGES-1) is an isozyme of the prostaglandin (PG) E synthase that acts downstream of cyclooxygenase and catalyzes the conversion of PGH2 to PGE2. The impact of genetic deletion of mPGES-1 on the development of 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis, a well-established model of inflammatory bowel disease (IBD), was investigated in this study. After administration of TNBS, mice deficient in mPGES-1 (mPGES-1-/- mice) showed more severe colitis than did wild-type (WT) mice. Histological examination revealed that mPGES-1-/- mice had markedly exacerbated symptoms of colitis. mPGES-1 expression was detectable in the colons of WT mice at both the mRNA and protein levels. Lack of mPGES-1 resulted in marked reduction of colonic PGE2 production. Our study also showed a significant increase in colonic expression of interleukin-17A (IL-17A), as well as interferon γ (IFNγ) and tumor necrosis factor α, during colitis in mPGES-1-/- mice compared with that in WT mice. Furthermore, loss of mPGES-1 increased the populations of IL-17A-producing T-helper (Th) 17 and IFNγ-producing Th1 cells in mesenteric lymph nodes. These results suggest that mPGES-1 is the main enzyme responsible for colonic PGE2 production and deficiency of mPGES-1 facilitates the development of colitis and T-cell-mediated immunity. mPGES-1 might, therefore, impact T-cell-related immune response associated with IBD.
Keywords: Th17 and Th1 cytokines; colitis; cyclooxygenase; inflammatory bowel disease; prostaglandin E synthase; prostaglandin E2.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Jakobsson P.J., Thoren S., Morgenstern R., Samuelsson B. Identification of human prostaglandin E synthase: A microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. USA. 1999;96:7220–7225. doi: 10.1073/pnas.96.13.7220. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- Integrative Research Program 2017-2018/Kitasato University Graduate School of Medical Sciences
- Student Research Program 2017-2018/Kitasato University Graduate School of Medical Sciences
- Student Research Program 2022-2023/Kitasato University Graduate School of Medical Sciences
- Research Program 2018/Regenerative Medicine and Cell Design Research Facility
- 2015-1033/Kitasato University School of Allied Health Sciences
LinkOut - more resources
Full Text Sources

