circARID1A Inhibits Tail Fat Cell Differentiation in Guangling Large-Tailed Sheep by Regulating the miR-493-3p/YTHDF2 Axis
- PMID: 39596416
- PMCID: PMC11594833
- DOI: 10.3390/ijms252212351
circARID1A Inhibits Tail Fat Cell Differentiation in Guangling Large-Tailed Sheep by Regulating the miR-493-3p/YTHDF2 Axis
Abstract
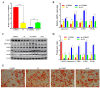
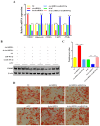
The Guangling Large-Tailed sheep is renowned for its unique tail fat deposition, with a significant proportion of its total body fat being localized in the tail region. Fat deposition is a complex biological process regulated by various molecular mechanisms. Our previous studies have identified a large number of differentially expressed circular RNAs (circRNAs) in the tail adipose tissue of the Guangling Large-Tailed sheep. These circRNAs may play a pivotal role in the process of fat deposition. Given the potential regulatory functions of circRNAs in adipose metabolism, investigating their roles in tail fat deposition is of significant scientific importance. In this study, we identified novel circARID1A. Using various experimental methods, including lentivirus infection, RNase R treatment, actinomycin D assay, qPCR, western blotting, and dual-luciferase reporter assays, we determined that circARID1A inhibits the expression of miR-493-3p through competitive binding, thereby regulating adipocyte differentiation. Further research revealed that miR-493-3p promotes adipocyte differentiation by targeting YTH domain family 2 (YTHDF2), and this regulatory effect is also influenced by circARID1A. In conclusion, our findings suggest that circARID1A inhibits tail fat cell differentiation in the Guangling Large-Tailed sheep through the circARID1A/miR-493-3p/YTHDF2 axis, providing theoretical support for improving meat quality and fat deposition in sheep.
Keywords: Guangling Large-Tailed sheep; YTHDF2; adipocyte differentiation; circARID1A; miR-493-3p.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Non-Coding RNAs in Regulating Fat Deposition in Farm Animals.Animals (Basel). 2025 Mar 11;15(6):797. doi: 10.3390/ani15060797. Animals (Basel). 2025. PMID: 40150326 Free PMC article. Review.
-
Elucidating the Role of circTIAM1 in Guangling Large-Tailed Sheep Adipocyte Proliferation and Differentiation via the miR-485-3p/PLCB1 Pathway.Int J Mol Sci. 2024 Apr 23;25(9):4588. doi: 10.3390/ijms25094588. Int J Mol Sci. 2024. PMID: 38731807 Free PMC article.
-
Multi-omics integrated analysis reveals the molecular mechanism of tail fat deposition differences in sheep with different tail types.BMC Genomics. 2025 May 9;26(1):465. doi: 10.1186/s12864-025-11658-y. BMC Genomics. 2025. PMID: 40346476 Free PMC article.
-
CircADAMTS16 Inhibits Differentiation and Promotes Proliferation of Bovine Adipocytes by Targeting miR-10167-3p.Cells. 2023 Apr 17;12(8):1175. doi: 10.3390/cells12081175. Cells. 2023. PMID: 37190084 Free PMC article.
-
microRNAs in the regulation of adipogenesis and obesity.Curr Mol Med. 2011 Jun;11(4):304-16. doi: 10.2174/156652411795677990. Curr Mol Med. 2011. PMID: 21506921 Free PMC article. Review.
Cited by
-
Non-Coding RNAs in Regulating Fat Deposition in Farm Animals.Animals (Basel). 2025 Mar 11;15(6):797. doi: 10.3390/ani15060797. Animals (Basel). 2025. PMID: 40150326 Free PMC article. Review.
References
-
- Zhang W., Xu M., Wang J., Wang S., Wang X., Yang J., Gao L., Gan S. Comparative Transcriptome Analysis of Key Genes and Pathways Activated in Response to Fat Deposition in Two Sheep Breeds with Distinct Tail Phenotype. Front. Genet. 2021;12:639030. doi: 10.3389/fgene.2021.639030. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

