Interaction Between Early Meals (Big-Breakfast Diet), Clock Gene mRNA Expression, and Gut Microbiome to Regulate Weight Loss and Glucose Metabolism in Obesity and Type 2 Diabetes
- PMID: 39596418
- PMCID: PMC11594859
- DOI: 10.3390/ijms252212355
Interaction Between Early Meals (Big-Breakfast Diet), Clock Gene mRNA Expression, and Gut Microbiome to Regulate Weight Loss and Glucose Metabolism in Obesity and Type 2 Diabetes
Abstract
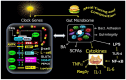
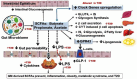
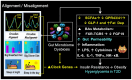
The circadian clock gene system plays a pivotal role in coordinating the daily rhythms of most metabolic processes. It is synchronized with the light-dark cycle and the eating-fasting schedule. Notably, the interaction between meal timing and circadian clock genes (CGs) allows for optimizing metabolic processes at specific times of the day. Breakfast has a powerful resetting effect on the CG network. A misaligned meal pattern, such as skipping breakfast, can lead to a discordance between meal timing and the endogenous CGs, and is associated with obesity and T2D. Conversely, concentrating most calories and carbohydrates (CH) in the early hours of the day upregulates metabolic CG expression, thus promoting improved weight loss and glycemic control. Recently, it was revealed that microorganisms in the gastrointestinal tract, known as the gut microbiome (GM), and its derived metabolites display daily oscillation, and play a critical role in energy and glucose metabolism. The timing of meal intake coordinates the oscillation of GM and GM-derived metabolites, which in turn influences CG expression, playing a crucial role in the metabolic response to food intake. An imbalance in the gut microbiota (dysbiosis) can also reciprocally disrupt CG rhythms. Evidence suggests that misaligned meal timing may cause such disruptions and can lead to obesity and hyperglycemia. This manuscript focuses on the reciprocal interaction between meal timing, GM oscillation, and circadian CG rhythms. It will also review studies demonstrating how aligning meal timing with the circadian clock can reset and synchronize CG rhythms and GM oscillations. This synchronization can facilitate weight loss and improve glycemic control in obesity and those with T2D.
Keywords: big-breakfast diet; circadian clock genes; gut microbiome; type 2 diabetes; weight loss.
Conflict of interest statement
The authors have no conflicts of interest.
Figures

Similar articles
-
Role of High Energy Breakfast "Big Breakfast Diet" in Clock Gene Regulation of Postprandial Hyperglycemia and Weight Loss in Type 2 Diabetes.Nutrients. 2021 May 5;13(5):1558. doi: 10.3390/nu13051558. Nutrients. 2021. PMID: 34063109 Free PMC article. Review.
-
Misalignment of Circadian Rhythms in Diet-Induced Obesity.Adv Exp Med Biol. 2024;1460:27-71. doi: 10.1007/978-3-031-63657-8_2. Adv Exp Med Biol. 2024. PMID: 39287848 Review.
-
Reduction in Glycated Hemoglobin and Daily Insulin Dose Alongside Circadian Clock Upregulation in Patients With Type 2 Diabetes Consuming a Three-Meal Diet: A Randomized Clinical Trial.Diabetes Care. 2019 Dec;42(12):2171-2180. doi: 10.2337/dc19-1142. Epub 2019 Sep 23. Diabetes Care. 2019. PMID: 31548244 Clinical Trial.
-
Differential roles of breakfast and supper in rats of a daily three-meal schedule upon circadian regulation and physiology.Chronobiol Int. 2011 Dec;28(10):890-903. doi: 10.3109/07420528.2011.622599. Chronobiol Int. 2011. PMID: 22080734
-
Influence of Fasting until Noon (Extended Postabsorptive State) on Clock Gene mRNA Expression and Regulation of Body Weight and Glucose Metabolism.Int J Mol Sci. 2023 Apr 12;24(8):7154. doi: 10.3390/ijms24087154. Int J Mol Sci. 2023. PMID: 37108316 Free PMC article. Review.
Cited by
-
Chrononutrition and Energy Balance: How Meal Timing and Circadian Rhythms Shape Weight Regulation and Metabolic Health.Nutrients. 2025 Jun 27;17(13):2135. doi: 10.3390/nu17132135. Nutrients. 2025. PMID: 40647240 Free PMC article. Review.
-
Fruit-Based Diet and Gut Health: A Review.Food Sci Nutr. 2025 Apr 30;13(5):e70159. doi: 10.1002/fsn3.70159. eCollection 2025 May. Food Sci Nutr. 2025. PMID: 40313793 Free PMC article. Review.
-
Chronotype and Cancer: Emerging Relation Between Chrononutrition and Oncology from Human Studies.Nutrients. 2025 Jan 31;17(3):529. doi: 10.3390/nu17030529. Nutrients. 2025. PMID: 39940387 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

