The Quenching of Long-Wavelength Fluorescence by the Closed Reaction Center in Photosystem I in Thermostichus vulcanus at 77 K
- PMID: 39596495
- PMCID: PMC11594324
- DOI: 10.3390/ijms252212430
The Quenching of Long-Wavelength Fluorescence by the Closed Reaction Center in Photosystem I in Thermostichus vulcanus at 77 K
Abstract
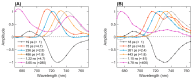
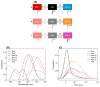
Photosystem I in most organisms contains long-wavelength or "Red" chlorophylls (Chls) absorbing light beyond 700 nm. At cryogenic temperatures, the Red Chls become quasi-traps for excitations as uphill energy transfer is blocked. One pathway for de-excitation of the Red Chls is via transfer to the oxidized RC (P700+), which has broad absorption in the near-infrared region. This study investigates the excitation dynamics of Red Chls in Photosystem I from the cyanobacterium Thermostichus vulcanus at cryogenic temperatures (77 K) and examines the role of the oxidized RC in modulating their fluorescence kinetics. Using time-resolved fluorescence spectroscopy, the kinetics of Red Chls were recorded for samples with open (neutral P700) and closed (P700+) RCs. We found that emission lifetimes in the range of 710-720 nm remained unaffected by the RC state, while more red-shifted emissions (>730 nm) decayed significantly faster when the RC was closed. A kinetic model describing the quenching by the oxidized RC was constructed based on simultaneous fitting to the recorded fluorescence emission in Photosystem I with open and closed RCs. The analysis resolved multiple Red Chl forms and variable quenching efficiencies correlated with their spectral properties. Only the most red-shifted Chls, with emission beyond 730 nm, are efficiently quenched by P700+, with rate constants of up to 6 ns-1. The modeling results support the notion that structural and energetic disorder in Photosystem I can have a comparable or larger effect on the excitation dynamics than the geometric arrangement of Chls.
Keywords: Thermosynechococcus vulcanus; chlorophyll; cyanobacteria; energy transfer; light harvesting; time-resolved fluorescence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Kinetically distinct three red chlorophylls in photosystem I of Thermosynechococcus elongatus revealed by femtosecond time-resolved fluorescence spectroscopy at 15 K.J Phys Chem B. 2010 Mar 4;114(8):2954-63. doi: 10.1021/jp909583r. J Phys Chem B. 2010. PMID: 20141149
-
Fluorescence spectroscopy of the longwave chlorophylls in trimeric and monomeric photosystem I core complexes from the cyanobacterium Spirulina platensis.Biochemistry. 1997 Nov 11;36(45):13830-7. doi: 10.1021/bi970386z. Biochemistry. 1997. PMID: 9374860
-
Excitation energy transfer kinetics of trimeric, monomeric and subunit-depleted Photosystem I from Synechocystis PCC 6803.Biochem J. 2021 Apr 16;478(7):1333-1346. doi: 10.1042/BCJ20210021. Biochem J. 2021. PMID: 33687054
-
Long-wavelength chlorophylls in photosystem I of cyanobacteria: origin, localization, and functions.Biochemistry (Mosc). 2014 Mar;79(3):213-20. doi: 10.1134/S0006297914030067. Biochemistry (Mosc). 2014. PMID: 24821447 Review.
-
Organization and role of the long-wave chlorophylls in the photosystem I of the Cyanobacterium spirulina.Membr Cell Biol. 1998;12(5):571-84. Membr Cell Biol. 1998. PMID: 10379641 Review.
References
-
- Karapetyan N.V., Schlodder E., van Grondelle R., Dekker J.P. The long-wavelength chlorophylls of photosystem I. In: Golbeck J.H., editor. Photosystem I: The Light-Driven, Plastocyanin:Ferredoxin Oxidoreductase. Springer; Dordrecht, The Netherlands: 2006. pp. 177–192.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

