ICOSLG Is Associated with Anti-PD-1 and Concomitant Antihistamine Treatment Response in Advanced Melanoma
- PMID: 39596506
- PMCID: PMC11594639
- DOI: 10.3390/ijms252212439
ICOSLG Is Associated with Anti-PD-1 and Concomitant Antihistamine Treatment Response in Advanced Melanoma
Abstract
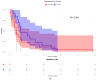
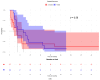
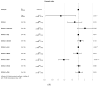
We previously demonstrated that patients with metastatic unresectable stage IIIb-IV melanoma receiving cetirizine (a second-generation H1 antagonist antihistamine) premedication with immunotherapy had better outcomes than those not receiving cetirizine. In this retrospective study, we searched for a gene signature potentially predictive of the response to the addition of cetirizine to checkpoint inhibition (nivolumab or pembrolizumab with or without previous ipilimumab). Transcriptomic analysis showed that inducible T cell costimulator ligand (ICOSLG) expression directly correlated with the disease control rate (DCR) when detected with a loading value > 0.3. A multivariable logistic regression model showed a positive association between the DCR and ICOSLG expression for progression-free survival and overall survival. ICOSLG expression was associated with CD64, a specific marker of M1 macrophages, at baseline in the patient samples who received cetirizine concomitantly with checkpoint inhibitors, but this association was not present in subjects who had not received cetirizine. In conclusion, our results show that the clinical advantage of concomitant treatment with cetirizine during checkpoint inhibition in patients with malignant melanoma is associated with high ICOSLG expression, which could predict the response to immune checkpoint inhibitor blockade.
Keywords: biomarker; cetirizine; checkpoint inhibition; cutaneous melanoma.
Conflict of interest statement
Alessandra Cesano and Sarah Warren were employed at ESSA Pharma. The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Sosman J.A., Atkins M.B., Leming P.D., et al. Five-Year Survival and Correlates Among Patients with Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated with Nivolumab. JAMA Oncol. 2019;5:1411–1420. doi: 10.1001/jamaoncol.2019.2187. - DOI - PMC - PubMed
-
- Robert C., Hwu W.J., Hamid O., Ribas A., Weber J.S., Daud A.I., Hodi F.S., Wolchok J.D., Mitchell T.C., Hersey P., et al. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: A landmark analysis in patients with advanced melanoma. Eur. J. Cancer. 2021;144:182–191. doi: 10.1016/j.ejca.2020.11.010. - DOI - PMC - PubMed
-
- Schachter J., Ribas A., Long G.V., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M., et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390:1853–1862. doi: 10.1016/S0140-6736(17)31601-X. - DOI - PubMed
-
- Versluis J.M., Menzies A.M., Sikorska K., Rozeman E.A., Saw R.P.M., van Houdt W.J., Eriksson H., Klop W.M.C., Ch’ng S., van Thienen J.V., et al. Survival update of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma in the OpACIN and OpACIN-neo trials. Ann. Oncol. 2023;34:420–430. doi: 10.1016/j.annonc.2023.01.004. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

