Effectiveness of Local Use of Green Propolis-Loaded Lipid Nanoparticles as Adjuvant Therapy to Scaling and Root Planing in the Management of Periodontitis in Rats Treated with Zoledronate
- PMID: 39596508
- PMCID: PMC11595208
- DOI: 10.3390/ijms252212443
Effectiveness of Local Use of Green Propolis-Loaded Lipid Nanoparticles as Adjuvant Therapy to Scaling and Root Planing in the Management of Periodontitis in Rats Treated with Zoledronate
Abstract
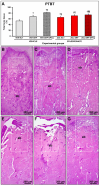
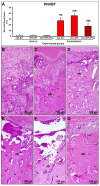
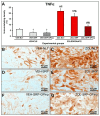
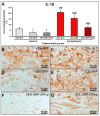
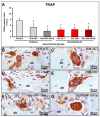
This study assessed the effectiveness of the local use of green propolis-loaded lipid nanoparticles (GPlnp) as an adjuvant therapy to scaling and root planing (SRP) to manage experimental periodontitis (EP) in ovariectomized rats treated with zoledronate. Ten weeks before the experiment, 48 female rats were ovariectomized. On day 0, a ligature was installed in the lower first molar to induce EP. From day 0 to day 42, half of the rats were treated with vehicle (VEH), while the other half were treated with 100μg/Kg of zoledronate (ZOL). On day 14, the rats were allocated into the following groups: VEH-NLT, VEH-SRP, VEH-SRP-GPlnp, ZOL-NLT, ZOL-SRP, and ZOL-SRP-GPlnp. VEH-NLT and ZOL-NLT received no local treatment. VEH-SRP and ZOL-SRP received SRP and irrigation with physiological saline solution. VEH-SRP-GPlnp and ZOL-SRP-GPlnp received SRP and irrigation with GPlnp. A single SRP session was carried out, and four irrigation sessions were conducted (on days 14, 16, 18, and 20). On day 42, all animals were euthanized. The hemimandibles were processed for histological, histometric (percentage of total bone tissue (PTBT) and non-vital bone tissue (PNVBT)) and immunohistochemical (TNFα, IL-1β, and TRAP) analysis. VEH-SRP-GPlnp showed better tissue repair, higher PTBT, and lower immunolabeling for TNFα and IL-1β compared to the groups treated with VEH. ZOL-SRP-GPlnp showed a favorable tissue repair, with lower PNVBT, less local inflammation, and lower immunolabeling for TNFα and IL-1β compared to the groups treated with ZOL. Irrigation with GPlnp proved to be effective as an adjuvant therapy to SRP in treating EP in ovariectomized rats treated with zoledronate.
Keywords: nanoparticles; periodontitis; propolis; scaling and root planing; zoledronate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Brazilian Green Propolis Carried in Lipid-Based Nanostructures: A Potent Adjuvant Therapy to Non-Surgical Periodontal Treatment in the Management of Experimental Periodontitis.Biomedicines. 2025 Jul 4;13(7):1643. doi: 10.3390/biomedicines13071643. Biomedicines. 2025. PMID: 40722716 Free PMC article.
-
Peri-implantitis increases the risk of medication-related osteonecrosis of the jaws associated with osseointegrated implants in rats treated with zoledronate.Sci Rep. 2024 Jan 5;14(1):627. doi: 10.1038/s41598-023-49647-4. Sci Rep. 2024. PMID: 38182598 Free PMC article.
-
Adjuvant Therapy With Sodium Alendronate for the Treatment of Experimental Periodontitis in Rats.J Periodontol. 2015 Oct;86(10):1166-75. doi: 10.1902/jop.2015.150166. Epub 2015 Jun 11. J Periodontol. 2015. PMID: 26062841
-
Influence of adjuvant therapy with green tea extract in the treatment of experimental periodontitis.Arch Oral Biol. 2019 Jun;102:65-73. doi: 10.1016/j.archoralbio.2019.03.028. Epub 2019 Apr 3. Arch Oral Biol. 2019. PMID: 30974379
-
Systemic doxycycline as an adjunct to scaling and root planing in diabetic patients with periodontitis: a systematic review and meta-analysis.BMC Oral Health. 2019 Sep 5;19(1):209. doi: 10.1186/s12903-019-0873-7. BMC Oral Health. 2019. PMID: 31488125 Free PMC article.
Cited by
-
Brazilian Green Propolis Carried in Lipid-Based Nanostructures: A Potent Adjuvant Therapy to Non-Surgical Periodontal Treatment in the Management of Experimental Periodontitis.Biomedicines. 2025 Jul 4;13(7):1643. doi: 10.3390/biomedicines13071643. Biomedicines. 2025. PMID: 40722716 Free PMC article.
References
-
- Ruggiero S.L., Dodson T.B., Aghaloo T., Carlson E.R., Ward B.B., Kademani D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral. Maxillofac. Surg. 2022;80:920–943. doi: 10.1016/j.joms.2022.02.008. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

