Exposure to Radiofrequency Electromagnetic Fields Enhances Melanin Synthesis by Activating the P53 Signaling Pathway in Mel-Ab Melanocytes
- PMID: 39596520
- PMCID: PMC11595227
- DOI: 10.3390/ijms252212457
Exposure to Radiofrequency Electromagnetic Fields Enhances Melanin Synthesis by Activating the P53 Signaling Pathway in Mel-Ab Melanocytes
Abstract
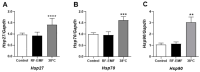
The skin is the largest body organ that can be physiologically affected by exposure to radiofrequency electromagnetic fields (RF-EMFs). We investigated the effect of RF-EMFs on melanogenesis; Mel-Ab melanocytes were exposed to 1760 MHz radiation with a specific absorption rate of 4.0 W/kg for 4 h/day over 4 days. Exposure to the RF-EMF led to skin pigmentation, with a significant increase in melanin production in Mel-Ab melanocytes. The phosphorylation level of cAMP response element binding protein (CREB) and the expression of microphthalmia-associated transcription factor (MITF), which regulate the expression of tyrosinase, were significantly increased in Mel-Ab after RF-EMF exposure. Interestingly, the expression of tyrosinase was significantly increased, but tyrosinase activity was unchanged in the RF-EMF-exposed Mel-Ab cells. Additionally, the expression of p53 and melanocortin 1 receptor (MC1R), which regulate MITF expression, was significantly increased. These results suggest that the RF-EMF induces melanogenesis by increasing phospho-CREB and MITF activity. Importantly, when Mel-Ab cells were incubated at 38 °C, the melanin production and the levels of tyrosinase significantly decreased, indicating that the increase in melanin synthesis by RF-EMF exposure is not due to a thermal effect. In conclusion, RF-EMF exposure induces melanogenesis in Mel-Ab cells through the increased expression of tyrosinase via the activation of MITF or the phosphorylation of CREB, which are initiated by the activation of p53 and MC1R.
Keywords: MC1R; MITF; melanogenesis; p53; phospho-CREB; radiofrequency electromagnetic fields; tyrosinase.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Puizina-Ivic N. Skin aging. Acta Dermatovenerol. Alp. Panon. Et Adriat. 2008;17:47. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

