Combating Tuberculosis via Restoring the Host Immune Capacity by Targeting M. tb Kinases and Phosphatases
- PMID: 39596546
- PMCID: PMC11595174
- DOI: 10.3390/ijms252212481
Combating Tuberculosis via Restoring the Host Immune Capacity by Targeting M. tb Kinases and Phosphatases
Abstract
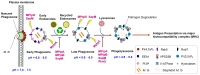
Mycobacterium tuberculosis (M. tb) is a remarkably versatile pathogen that possesses a unique ability to counteract the host's defence mechanisms to control the infection. Several mycobacterial protein kinases and phosphatases were found to play a key role in impeding phagosome maturation in macrophages and accordingly blocking the phagosome-lysosome fusion, therefore allowing the bacteria to survive. During phagocytosis, both M. tb and the host's phagocytic cells develop mechanisms to fight each other, resulting in pathogen elimination or survival. In this respect, M. tb uses a phosphorylation-based signal transduction mechanism, whereby it senses extracellular signals from the host and initiates the appropriate adaptation responses. Indeed, the ability of M. tb to exist in different states in the host (persistent quiescent state or actively replicating mode) is mainly mediated through protein phosphorylation/dephosphorylation signalling. The M. tb regulatory and defensive responses coordinate different aspects of the bacilli's physiology, for instance, cell wall components, metabolic activity, virulence, and growth. Herein, we will discuss the implication of M. tb kinases and phosphatases in hijacking the host immune system, perpetuating the infection. In addition, the role of PknG, MPtpA, MPtpB, and SapM inhibitors in resetting the host immune system will be highlighted.
Keywords: MPtpA; MPtpB; PknG; SapM; host immune response; mycobacterial kinase inhibitor; mycobacterial kinases; mycobacterial phosphatase inhibitor; mycobacterial phosphatases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

