Safety and Efficacy of Dalbavancin in Real Life: Retrospective Analysis of a Large Monocentric Case Series of Patients Treated for Skin/Soft Tissue and Other Difficult-to-Treat Infections
- PMID: 39596758
- PMCID: PMC11591112
- DOI: 10.3390/antibiotics13111063
Safety and Efficacy of Dalbavancin in Real Life: Retrospective Analysis of a Large Monocentric Case Series of Patients Treated for Skin/Soft Tissue and Other Difficult-to-Treat Infections
Abstract
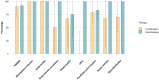
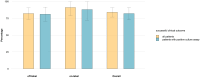
Background: Dalbavancin is a long-acting lipoglycopeptide, approved for treatment of skin and skin structure infections. Its PK/PD profile and safety allow for short hospital stays even in the case of difficult-to-treat infections requiring long courses of therapy, e.g., osteomyelitis, cardiovascular, and prosthetic infections. Objectives: We aimed to evaluate the safety and efficacy of dalbavancin in real life settings for both in-label and off-label indications. Methods: retrospective evaluation of all consecutive patients treated with dalbavancin at our site between May 2017 and September 2021. Results: A total of 100 patients treated with dalbavancin and followed up for 6 months after treatment (58% male; median age 63.5 years, median Charlson Comorbidity Index CCI = 2.7, 28% inpatients) were included with the following indications: acute bacterial skin and skin structure infections (22%), bone and prosthetic infections (57%), and cardiovascular infections (19%). Infections were caused by MSSA (30%), MRSA (5%), MR-CoNS (20%), and Streptococcus spp. (8%). In 32 cases, no isolate was obtained. The average number of infusions was 5 (s.d. = 3). Neither ensuing alteration of renal function nor neutropenia or thrombocytopenia were observed during treatment and follow-up. Two self-limiting skin rashes occurred. The overall clinical success rate was 84%-91% for registered and 82% for unregistered indications. The prescription of higher loading doses was the only predictor independently associated with better outcomes in multivariate models (OR: 5.2, 95%CI: 1.5-17.9, p < 0.01). Conclusions: Dalbavancin proved to be effective for skin and skin structure infections, as well as for difficult-to-treat infections in highly comorbid patients. Regarding tolerability, our results support the use of dalbavancin for long-lasting treatments of deep-seated infections.
Keywords: Gram-positive infections; antibiotic therapy; dalbavancin; infections; tolerability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Cojutti P.G., Rinaldi M., Zamparini E., Rossi N., Tedeschi S., Conti M., Pea F., Viale P. Population Pharmacokinetics of Dalbavancin and Dosing Consideration for Optimal Treatment of Adult Patients with Staphylococcal Osteoarticular Infections. Antimicrob. Agents Chemother. 2023;65:e02260-20. doi: 10.1128/AAC.02260-20. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous