Comparison of Prognostic Values of Seven Immune Indexes in Advanced Non-Small-Cell Lung Cancer Treated with Nivolumab: How Effective Can They Be Regarding Our Treatment Decisions?
- PMID: 39596977
- PMCID: PMC11596302
- DOI: 10.3390/medicina60111792
Comparison of Prognostic Values of Seven Immune Indexes in Advanced Non-Small-Cell Lung Cancer Treated with Nivolumab: How Effective Can They Be Regarding Our Treatment Decisions?
Abstract
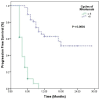
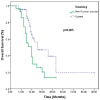
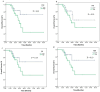
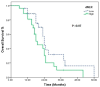
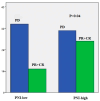
Background and Objectives: In this study, we evaluated the impact of seven immune indexes on treatment response and survival outcomes in advanced non-small-cell lung cancer (NSCLC) patients receiving second-line and subsequent nivolumab treatment under real-life conditions. Materials and Methods: The pan-immune inflammation value (PIV), systemic immune inflammation value (SII), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), derived neutrophil-to-lymphocyte ratio (d-NLR), and prognostic nutritional index (PNI) were calculated. All immune indexes were classified as low and high based on cut-off values. Kaplan-Meier and Cox hazard models were used for survival analysis. Results: The median follow-up was 22.0 months (6.0-96.0). The median overall survival (OS) was 30.0 months and the median progression-free survival (PFS) was 7.0 months. In the univariate analysis, comorbidity (p = 0.03) and nivolumab use for more than eight cycles (p < 0.0001) were associated with an increase in PFS, while smoking history (p < 0.005) and d-NLR (p < 0.05) were more effective regarding OS. Patients who received more than eight cycles of nivolumab had longer median PFS (4 vs. 19 months, p < 0.001) and OS (23 vs. 43 months, p < 0.001). We found longer median OS in the PLR (45.7 vs. 75.4 months; p = 0.05), PIV (53.0 vs. 66.4 months; p = 0.19), SII (50.0 vs. 71.9 vs. months, p = 0.19), and NLR (49.9 vs. 74.55 months, p = 0.10) indexes in nivolumab long-term users (high vs. low groups, respectively). In short-term users of nivolumab, only d-NLR median OS (high vs. low, 19 vs. 75.2 months, p = 0.07) was different. Complete and partial response rates to nivolumab treatment were higher in the PNI-high group (p = 0.04). Conclusions: In these real-life data, we determined that the PLR, PIV, SII, and NLR indexes were effective in the prognosis of patients who received PD1 inhibitor nivolumab for a long time, and the d-NLR index was effective in those who developed progression in a short time. We found that the PNI was effective in patients who responded well to ICI treatment.
Keywords: derived neutrophil-to-lymphocyte ratio (d-NLR); monocyte-to-lymphocyte ratio (MLR); neutrophil-to-lymphocyte ratio (NLR); nivolumab; pan-immune inflammation value (PIV); platelet-to-lymphocyte ratio (PLR); prognostic nutritional index (PNI); systemic immune inflammation value (SII).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Kazandjian D., Suzman D.L., Blumenthal G., Mushti S., He K., Libeg M., Keegan P., Pazdur R. FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer with Progression on or After Platinum-Based Chemotherapy. Oncologist. 2016;21:634–642. doi: 10.1634/theoncologist.2015-0507. - DOI - PMC - PubMed
-
- Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

