Revisiting Socransky's Complexes: A Review Suggesting Updated New Bacterial Clusters (GF-MoR Complexes) for Periodontal and Peri-Implant Diseases and Conditions
- PMID: 39597602
- PMCID: PMC11596145
- DOI: 10.3390/microorganisms12112214
Revisiting Socransky's Complexes: A Review Suggesting Updated New Bacterial Clusters (GF-MoR Complexes) for Periodontal and Peri-Implant Diseases and Conditions
Abstract
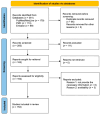
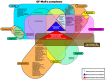
This review aimed to identify newly discovered bacteria from individuals with periodontal/peri-implant diseases and organize them into new clusters (GF-MoR complexes) to update Socransky's complexes (1998). For methodological development, the PCC (Population, Concept, Context) strategy was used for the focus question construction: "In patients with periodontal and/or peri-implant disease, what bacteria (microorganisms) were detected through laboratory assays?" The search strategy was applied to PubMed/MEDLINE, PubMed Central, and Embase. The search key terms, combined with Boolean markers, were (1) bacteria, (2) microbiome, (3) microorganisms, (4) biofilm, (5) niche, (6) native bacteria, (7) gingivitis), (8) periodontitis, (9) peri-implant mucositis, and (10) peri-implantitis. The search was restricted to the period 1998-2024 and the English language. The bacteria groups in the oral cavity obtained/found were retrieved and included in the GF-MoR complexes, which were based on the disease/condition, presenting six groups: (1) health, (2) gingivitis, (3) peri-implant mucositis, (4) periodontitis, (5) peri-implantitis, and (6) necrotizing and molar-incisor (M-O) pattern periodontitis. The percentual found per group refers to the number of times a specific bacterium was found to be associated with a particular disease. A total of 381 articles were found: 162 articles were eligible for full-text reading (k = 0.92). Of these articles, nine were excluded with justification, and 153 were included in this review (k = 0.98). Most of the studies reported results for the health condition, periodontitis, and peri-implantitis (3 out of 6 GF-MoR clusters), limiting the number of bacteria found in the other groups. Therefore, it became essential to understand that bacterial colonization is a dynamic process, and the bacteria present in one group could also be present in others, such as those observed with the bacteria found in all groups (Porphyromonas gingivalis, Tannarela forsythia, Treponema denticola, and Aggregatibacter actinomycetemcomitans) (GF-MoR's red triangle). The second most observed bacteria were grouped in GF-MoR's blue triangle: Porphyromonas spp., Prevotela spp., and Treponema spp., which were present in five of the six groups. The third most detected bacteria were clustered in the grey polygon (GF-MoR's grey polygon): Fusobacterium nucleatum, Prevotella intermedia, Campylobacter rectus, and Eikenella corrodens. These three geometric shapes had the most relevant bacteria to periodontal and peri-implant diseases. Specifically, per group, GF-MoR's health group had 58 species; GF-MoR's gingivitis group presented 16 bacteria; GF-MoR's peri-implant mucositis included 17 bacteria; GF-MoR's periodontitis group had 101 different bacteria; GF-MoR's peri-implantitis presented 61 bacteria; and the last group was a combination of necrotizing diseases and molar-incisor (M-I) pattern periodontitis, with seven bacteria. After observing the top seven bacteria of all groups, all of them were found to be gram-negative. Groups 4 and 5 (periodontitis and peri-implantitis) presented the same top seven bacteria. For the first time in the literature, GF-MoR's complexes were presented, gathering bacteria data according to the condition found and including more bacteria than in Socransky's complexes. Based on this understanding, this study could drive future research into treatment options for periodontal and peri-implant diseases, guiding future studies and collaborations to prevent and worsen systemic conditions. Moreover, it permits the debate about the evolution of bacterial clusters.
Keywords: Socransky’s complexes; bacteria; bacterium; clusters; complexes; gingivitis; newly identified peri-implant pathogens; newly identified periodontal pathogens; peri-implant mucositis; peri-implantitis; periodontal health; periodontitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Fernandes G.V.O., Akman A.C., Hakki S.S. Periodontal therapy for hopeless mandibular anterior teeth: A retrospective case report with a multidisciplinary approach and a 20-year follow-up. Int. J. Sci. Dent. 2024;66:1–11.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

