The Antiviral Effects of Heat-Killed Lactococcus lactis Strain Plasma Against Dengue, Chikungunya, and Zika Viruses in Humans by Upregulating the IFN-α Signaling Pathway
- PMID: 39597693
- PMCID: PMC11596828
- DOI: 10.3390/microorganisms12112304
The Antiviral Effects of Heat-Killed Lactococcus lactis Strain Plasma Against Dengue, Chikungunya, and Zika Viruses in Humans by Upregulating the IFN-α Signaling Pathway
Abstract
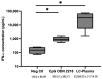
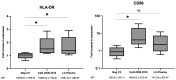
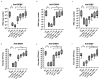
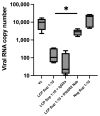
The growing risk of contracting viral infections due to high-density populations and ecological disruptions, such as climate change and increased population mobility, has highlighted the necessity for effective antiviral treatment and preventive measures against Dengue virus (DENV), Chikungunya virus (CHIKV), and Zika virus (ZIKV). Recently, there has been increasing attention on the use of probiotics as a potential antiviral option to reduce virus infections. The present study aimed to assess the immunomodulatory effects of heat-killed Lactococcus lactis strain plasma (LC-Plasma) on peripheral blood mononuclear cells (PBMCs) and its subsequent antiviral response against DENV, CHIKV, and ZIKV. To evaluate the immunomodulatory effects of LC-Plasma on PBMCs isolated from healthy individuals, PBMCs were cultured at a density of 2 × 105 cells/well and stimulated with 10 µg/mL of LC-Plasma. LC-plasma-stimulated PBMCs demonstrated elevated interferon-alpha (IFN-α) production and cluster of differentiation 86 (CD86) and human leukocyte antigen-DR isotype (HLA-DR) upregulation, potentially linked to plasmacytoid dendritic cell (pDC) activation. The replication of DENV, CHIKV, and ZIKV was dose-dependently inhibited when Huh-7 cells were stimulated with LC-Plasma-stimulated PBMC supernatant (LCP Sup). IFN-stimulated gene (ISG) expression, including IFN-stimulated gene 15 (ISG15), IFN-stimulated exonuclease gene 20 (ISG20), IFN-induced transmembrane protein 1 (IFITM-1), myxovirus resistance protein A (MxA), and radical S-adenosyl methionine domain-containing protein 2 (RSAD2), was significantly upregulated in LCP Sup-stimulated Huh-7 cells. Findings from this study indicate that LC-Plasma has the potential to induce IFN-α production, leading to an enhancement in the expression of ISGs and contributing to a broad-spectrum antiviral response. Thus, LC-Plasma may serve as a rational adjunctive treatment to ameliorate viral diseases, warranting future clinical trials.
Keywords: LC-Plasma; antiviral; arboviruses; interferon; probiotics.
Conflict of interest statement
The authors declare that this study received funding from Institute of Health Sciences, Kirin Holdings Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures

References
-
- Chikungunya Worldwide Overview. [(accessed on 6 February 2024)]. Available online: https://www.ecdc.europa.eu/en/chikungunya-monthly.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

