German Real-World Experience of Patients with Diverse Features of Acute Intermittent Porphyria Treated with Givosiran
- PMID: 39597922
- PMCID: PMC11594983
- DOI: 10.3390/jcm13226779
German Real-World Experience of Patients with Diverse Features of Acute Intermittent Porphyria Treated with Givosiran
Abstract
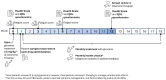
Background/Objectives: Acute intermittent porphyria (AIP) is a metabolic disease characterised by neurovisceral crises with episodes of acute abdominal pain alongside life-altering, and often hidden, chronic symptoms. The elimination of precipitating factors, hemin therapy, and pain relief are strategies used to treat porphyria symptoms, but are often reserved for patients suffering recurrent, acute attacks. Givosiran (siRNA) is an emerging AIP therapy capable of silencing delta-aminolevulinic acid synthase-1 (ALAS1) and, in turn, reducing the accumulation of delta-aminolevulinic acid (ALA) and porphobilinogen (PBG) that precede porphyria symptoms. The aim of this study was to investigate the efficacy and safety of givosiran administration in patients with both acute and chronic AIP burden, who were poorly responsive to current therapies, using a personalised medicine approach. Methods: Real-world data were collected in consecutive patients treated with givosiran at an accredited German Porphyria Clinical Center. Biochemical, clinical, and HR-QoL outcomes were monitored alongside adverse events (AEs). Results: Twenty-eight patients treated between 2018 and 2024 were sub-categorised into groups corresponding to Ipnet terms 13 'Sporadic Attacks, 5 'Symptomatic High Excretors', 5 'Prophylactic Heme', and 5 "Recurrent Attacks'. The mean time from diagnosis to treatment was 9.2 years (range in months 1-324), and the mean duration of treatment was 30 months (range 3-68). After 6 months of monthly givosiran injection (2.5 mg/kg), all patients' ALA levels reached <2ULN, and 60% of patients attained PBG levels < 2ULN (p < 0.001). These biochemical responses were not different between sub-groups (p > 0.05). Clinically, 75% of patients' chronic and acute porphyria symptoms improved. The total patient populations' annualised attack ratio (AAR) improved; Historical AAR: 2.9 (0-12.0) vs. Givo AAR: 0.45 (0-3.0) (p < 0.01). During follow-up, nine patients experienced minor breakthrough episodes. Of these, three patients required hemin infusion. An association between clinical success and a shorter interim period between diagnosis and treatment was evident (r = -0.522, p = 0.0061). All patients' indices of HR-QoL improved under givosiran, including mental health (38%, p < 0.0001) and pain (38%, p < 0.0001). Patient-reported health (givosiran 77.9% vs. baseline 37.1%, p < 0.0001) and clinical outcome scores (86.9%: good-very good) were also positive. Two patients withdrew from treatment <6 months, citing fatigue, which was a common side effect. A mild elevation in liver enzymes (AST and/or ALT < 1.5ULN, 15.4%) and reduced glomerular filtration rates (GFR, 11.5%) were also evident, but no life-threatening adverse events (AEs) were attributed to givosiran treatment. Conclusions: Givosiran is effective in preventing severe acute attacks and reducing the chronic health burden in patients with acute intermittent porphyria. Importantly, HR-QoL improved in patients suffering chronic AIP burden with few incidences of historical attacks. All patients experienced substantially improved mental health, ease of living, and self-perceived health.
Keywords: AHP; AIP; ALA; ALAS1; Fatigue Assessment Scale (FAS); HR-QoL; Ipnet; PBG; chronic symptoms; fatigue; givosiran; siRNA; sporadic attack.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







References
-
- Dayan F., Dayan E. Porphyrins: One Ring in the Colors of Life. Am. Sci. 2011;99:236. doi: 10.1511/2011.90.236. - DOI
-
- Deybach J.-C., Badminton M., Puy H., Sandberg S., Frank J., Harper P., Martasek P., Minder E., Parker S., Thunell S., et al. European Porphyria Initiative (EPI): A Platform to Develop a Common Approach to the Management of Porphyrias and to Promote Research in the Field. Physiol. Res. 2006;55:S67–S73. doi: 10.33549/physiolres.930000.55.S2.67. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

