MRI Spinal Cord Reconstruction Provides Insights into Mapping and Migration Following Percutaneous Epidural Stimulation Implantation in Spinal Cord Injury
- PMID: 39597970
- PMCID: PMC11594936
- DOI: 10.3390/jcm13226826
MRI Spinal Cord Reconstruction Provides Insights into Mapping and Migration Following Percutaneous Epidural Stimulation Implantation in Spinal Cord Injury
Abstract
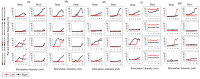
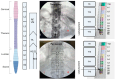
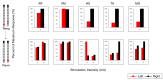
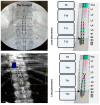
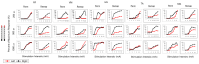
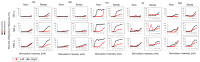
Background: Spinal cord epidural stimulation (SCES) has the potential to restore motor functions following spinal cord injury (SCI). Spinal cord mapping is a cornerstone step towards successfully configuring SCES to improve motor function, aiming to restore standing and stepping abilities in individuals with SCI. While some centers have advocated for the use of intraoperative mapping to anatomically target the spinal cord locomotor centers, this is a resource-intensive endeavor and may not be a feasible approach in all centers. Methods: Two participants underwent percutaneous SCES implantation as part of a clinical trial. Each participant underwent a temporary (1-week, two-lead) trial followed by a permanent, two-lead implantation. SCES configurations were matched between temporary and permanent mappings, and motor evoked potential in response to 2 Hz, for a duration of 250-1000 µs and with an amplitude of 1-14 mA, was measured using electromyography. T2 axial MRI images captured prior to implantation were used to retrospectively reconstruct the lumbosacral segments of the spinal cord. The effects of lead migration on mapping were further determined in one of the participants. Results: In both participants, there were recognized discrepancies in the recruitment curves of the motor evoked potentials across different muscle groups between temporary and permanent SCES mappings. These may be explained by retrospective MRI reconstruction of the spinal cord, which indicated that the percutaneous leads did not specifically target the entire L1-S2 segments in both participants. Minor lead migration appeared to have a minimal impact on spinal cord mapping outcomes in one of the participants but did dampen the motor activity of the hip and knee muscle groups. Conclusions: Temporary mapping coupled with MRI reconstruction has the potential to be considered as guidance for permanent implantation considering target activation of the spinal cord locomotor centers. Since lead migration may alter the synergistic coordination between different muscle groups and since lead migration of 1-2 contacts is expected and planned for in clinical practice, it can be better guided with proper spinal cord mapping and a diligent SCES lead trial beforehand.
Keywords: MRI; permanent mapping; spinal cord epidural stimulation (SCES); spinal cord injury; spinal cord reconstruction; temporary mapping.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Anjum A., Yazid M.D., Fauzi Daud M., Idris J., Ng A.M.H., Selvi Naicker A., Ismail O.H.R., Athi Kumar R.K., Lokanathan Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020;21:7533. doi: 10.3390/ijms21207533. - DOI - PMC - PubMed
-
- Pang Q.M., Chen S.Y., Xu Q.J., Fu S.P., Yang Y.C., Zou W.H., Zhang M., Liu J., Wan W.H., Peng J.C., et al. Neuroinflammation and Scarring After Spinal Cord Injury: Therapeutic Roles of MSCs on Inflammation and Glial Scar. Front. Immunol. 2021;12:751021. doi: 10.3389/fimmu.2021.751021. - DOI - PMC - PubMed
-
- Turczyn P., Wojdasiewicz P., Poniatowski Ł.A., Purrahman D., Maślińska M., Żurek G., Romanowska-Próchnicka K., Żuk B., Kwiatkowska B., Piechowski-Jóźwiak B., et al. Omega-3 fatty acids in the treatment of spinal cord injury: Untapped potential for therapeutic intervention? Mol. Biol. Rep. 2022;49:10797–10809. doi: 10.1007/s11033-022-07762-x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

