Nephro- and Cardiotoxic Effects of Etoricoxib: Insights into Arachidonic Acid Metabolism and Beta-Adrenergic Receptor Expression in Experimental Mice
- PMID: 39598366
- PMCID: PMC11597224
- DOI: 10.3390/ph17111454
Nephro- and Cardiotoxic Effects of Etoricoxib: Insights into Arachidonic Acid Metabolism and Beta-Adrenergic Receptor Expression in Experimental Mice
Abstract
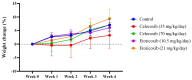
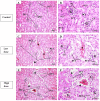
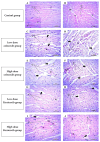
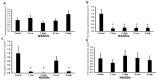
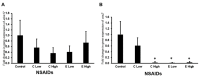
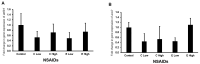
Background: Etoricoxib is a widely used anti-inflammatory drug, but its safety profile concerning cardiovascular and renal health remains inadequately explored. This study aimed to assess the nephro- and cardiotoxic effects of etoricoxib in a murine model, with a focus on its impact on arachidonic acid-metabolizing enzymes and beta-adrenergic receptors associated with drug-induced toxicity. Methods: Thirty-five BALB/C mice were randomly assigned to five groups: control, low-dose etoricoxib, high-dose etoricoxib, low-dose celecoxib, and high-dose celecoxib (a well-known nephro- and cardiotoxic NSAID). The treatments were administered for 28 days, after which hearts and kidneys were excised for physical and histopathological analysis, and the expression of arachidonic acid-metabolizing enzymes (cytochrome P450s, lipoxygenases, cyclooxygenases) and beta-1 adrenergic receptor (adrb1) and angiotensin-converting enzyme (ace2) genes were quantified using quantitative reverse transcription PCR (qRT-PCR). Results: Etoricoxib administration resulted in dose-dependent nephro- and cardiotoxic effects. Renal histology revealed glomerular atrophy or hypertrophy and significant damage to the proximal and distal convoluted tubules, including epithelial flattening, cytoplasmic vacuolation, and luminal widening. Cardiac analysis showed disorganized muscle fibers and hyaline degeneration. These changes were associated with altered gene expression: the downregulation of cox2, cyp1a1, and cyp2c29 in the kidneys and the upregulation of cyp4a12, cox2, and adrb1, along with the downregulation of cyp2c29 and ace2 in the heart. Conclusions: Etoricoxib induces nephro- and cardiotoxicity, marked by alterations in arachidonic acid metabolism and beta-adrenergic signaling pathways. The drug affects the expression of arachidonic acid-metabolizing enzymes and adrb1 in the heart while downregulating cox2 and other related enzymes in the kidneys. These findings underscore the need for caution when prescribing etoricoxib, particularly in patients with pre-existing renal or cardiac conditions.
Keywords: arachidonic acid; cardiotoxicity; cytochrome P450s; etoricoxib; nephrotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- De Vecchis R., Baldi C., Di Biase G., Ariano C., Cioppa C., Giasi A., Valente L., Cantatrione S. Cardiovascular risk associated with celecoxib or etoricoxib: A meta-analysis of randomized controlled trials which adopted comparison with placebo or naproxen. Minerva Cardioangiol. 2014;62:437–448. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

