The Impact of COVID-19 on RNA Therapeutics: A Surge in Lipid Nanoparticles and Alternative Delivery Systems
- PMID: 39598489
- PMCID: PMC11597542
- DOI: 10.3390/pharmaceutics16111366
The Impact of COVID-19 on RNA Therapeutics: A Surge in Lipid Nanoparticles and Alternative Delivery Systems
Abstract
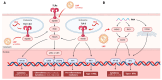
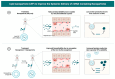
The COVID-19 pandemic has significantly accelerated progress in RNA-based therapeutics, particularly through the successful development and global rollout of mRNA vaccines. This review delves into the transformative impact of the pandemic on RNA therapeutics, with a strong focus on lipid nanoparticles (LNPs) as a pivotal delivery platform. LNPs have proven to be critical in enhancing the stability, bioavailability, and targeted delivery of mRNA, facilitating the unprecedented success of vaccines like those developed by Pfizer-BioNTech and Moderna. Beyond vaccines, LNP technology is being explored for broader therapeutic applications, including treatments for cancer, rare genetic disorders, and infectious diseases. This review also discusses emerging RNA delivery systems, such as polymeric nanoparticles and viral vectors, which offer alternative strategies to overcome existing challenges related to stability, immune responses, and tissue-specific targeting. Additionally, we examine the pandemic's influence on regulatory processes, including the fast-tracked approvals for RNA therapies, and the surge in research funding that has spurred further innovation in the field. Public acceptance of RNA-based treatments has also grown, laying the groundwork for future developments in personalized medicine. By providing an in-depth analysis of these advancements, this review highlights the long-term impact of COVID-19 on the evolution of RNA therapeutics and the future of precision drug delivery technologies.
Keywords: COVID-19 vaccines; RNA therapeutics; drug delivery systems; lipid nanoparticles; mRNA technology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

