Treatment of Inflammatory Bowel Disease by Using Curcumin-Containing Self-Microemulsifying Delivery System: Macroscopic and Microscopic Analysis
- PMID: 39598530
- PMCID: PMC11597465
- DOI: 10.3390/pharmaceutics16111406
Treatment of Inflammatory Bowel Disease by Using Curcumin-Containing Self-Microemulsifying Delivery System: Macroscopic and Microscopic Analysis
Erratum in
-
Correction: Ameer et al. Treatment of Inflammatory Bowel Disease by Using Curcumin-Containing Self-Microemulsifying Delivery System: Macroscopic and Microscopic Analysis. Pharmaceutics 2024, 16, 1406.Pharmaceutics. 2025 Jun 23;17(7):810. doi: 10.3390/pharmaceutics17070810. Pharmaceutics. 2025. PMID: 40733156 Free PMC article.
Abstract
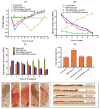
Background: The lack of local availability for drugs in the colon can be addressed by preparing a self-microemulsifying drug delivery system (SMEDDS) of curcumin (Cur) which is ultimately used for the treatment of inflammatory bowel disease (IBD). Methods: From preformulation studies, Lauroglycol FCC (oil), Tween 80 (surfactant), Transcutol HP (co-surfactant), and Avicel (solid carrier) were selected for the preparation of blank liquid and solid Cur-loaded SMEDDSs (S-Cur-SMEDDSs). Results: Z-average size (12.36 ± 0.04 nm), zeta potential (-14.7 ± 0.08 mV), and polydispersity index (PDI) (0.155 ± 0.036) showed a comparative droplet surface area and charge of both SMEDDSs. The physicochemical stability of Cur in S-Cur-SMEDDSs was confirmed via FTIR, DSC, TGA, and XRD analyses, while morphological analysis through SEM and atomic force microscopy (AFM) confirmed Cur loading into SMEDDSs with an increased surface roughness root mean square (RMS) of 11.433 ± 0.91 nm, greater than the blank SMEDDS. Acute toxicity studies with an organ weight ratio and % hemolysis of 15.65 ± 1.32% at a high concentration of 600 mM showed that S-Cur-SMEDDSs are safe at a medium dose (0.2-0.8 g/kg/day). The excellent in vitro antioxidant (68.54 ± 1.42%) and anti-inflammatory properties (56.47 ± 1.17%) of S-Cur-SMEDDS proved its therapeutic efficacy for IBD. Finally, S-Cur-SMEDDS significantly improved acetic acid-induced IBD in albino rats through a reduction in the disease activity index (DAI) and macroscopic ulcer score (MUS) from 4.15 ± 0.21 to 1.62 ± 0.12 at 15 mg/kg/day dose, as confirmed via histopathological assay. Conclusions: Based on the above findings, S-Cur-SMEDDS appears to be a stable, less toxic, and more efficacious alternative for Cur delivery with strong competence in treating IBD.
Keywords: acute toxicity; colon; curcumin; inflammatory bowel disease; self-microemulsifying drug delivery system.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Singh S., Yadav K., Varma A.K. Inflammatory Bowel Disease: An Overview of Immune Mechanisms and Biological Treatments. Int. J. Pharm. Life Sci. 2019;10:32.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

