Amphiphilic Oligonucleotide Derivatives-Promising Tools for Therapeutics
- PMID: 39598570
- PMCID: PMC11597563
- DOI: 10.3390/pharmaceutics16111447
Amphiphilic Oligonucleotide Derivatives-Promising Tools for Therapeutics
Abstract
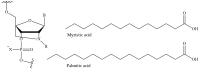
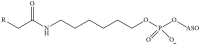
Recent advances in genetics and nucleic acid chemistry have created fundamentally new tools, both for practical applications in therapy and diagnostics and for fundamental genome editing tasks. Nucleic acid-based therapeutic agents offer a distinct advantage of selectively targeting the underlying cause of the disease. Nevertheless, despite the success achieved thus far, there remain unresolved issues regarding the improvement of the pharmacokinetic properties of therapeutic nucleic acids while preserving their biological activity. In order to address these challenges, there is a growing focus on the study of safe and effective delivery methods utilising modified nucleic acid analogues and their lipid bioconjugates. The present review article provides an overview of the current state of the art in the use of chemically modified nucleic acid derivatives for therapeutic applications, with a particular focus on oligonucleotides conjugated to lipid moieties. A systematic analysis has been conducted to investigate the ability of amphiphilic oligonucleotides to self-assemble into micelle-like structures, as well as the influence of non-covalent interactions of such derivatives with serum albumin on their biodistribution and therapeutic effects.
Keywords: amphiphilic oligonucleotides; modified nucleic acids; nucleic acid self-assembly; protein–oligonucleotide complexes; serum albumin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







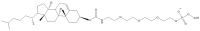

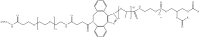




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

