Vibrational Spectroscopic Identification of the [AlCl2]+ Cation in Ether-Containing Liquid Electrolytes
- PMID: 39598766
- PMCID: PMC11597026
- DOI: 10.3390/molecules29225377
Vibrational Spectroscopic Identification of the [AlCl2]+ Cation in Ether-Containing Liquid Electrolytes
Abstract
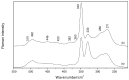
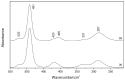
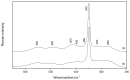
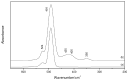
A Raman and IR study of AlCl3-based ethereal solutions is here presented and aims at identifying the [AlCl2]+ cation, which has been so far unambiguously characterized by 27Al NMR spectrometry. To do that, experimental-theoretical vibrational spectroscopy was so employed, and the data are interpreted successfully. As a known amount of water is added to the tetrahydrofuran (THF)-containing electrolyte, a Raman band at 271 cm-1 has its intensity increased along with the most intense band of [AlCl4]-, and such behavior is also seen for a band at 405 cm-1 in the IR spectra. New bands at around 420 and 400 cm-1 are observed in both Raman and IR spectra for the tetraglyme (G4)-based systems. The [AlCl2(THF)4]+ complex, in the cis and trans forms, is present in the cyclic ether, while the cis-[AlCl2(G4)]+ isomer is identified in the acyclic one.
Keywords: aluminum chloride; ethereal liquid electrolytes; vibrational spectroscopy.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Couch D.E., Brenner A. A Hydride Bath for the Electrodeposition of Aluminum. J. Electrochem. Soc. 1952;99:234–244. doi: 10.1149/1.2779711. - DOI
-
- Eilmes A., Alves W.A. Combining experimental and theoretical vibrational spectroscopy to study magnesium aluminum chloride complex electrolytes. J. Mol. Liq. 2021;333:116053. doi: 10.1016/j.molliq.2021.116053. - DOI
-
- Eilmes A., Alves W.A. Theory-experiment partnership applied to the spectroscopic analysis of a promising conditioning-free electrolyte for Mg batteries. J. Mol. Liq. 2022;350:118528. doi: 10.1016/j.molliq.2022.118528. - DOI
-
- Eilmes A., Alves W.A. Unraveling the solvates in a diethylene glycol dimethyl ether-based electrolyte: A computational-experimental spectroscopic contribution to Mg battery area. J. Mol. Liq. 2022;359:119251. doi: 10.1016/j.molliq.2022.119251. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

