Insights into SARS-CoV-2: Small-Molecule Hybrids for COVID-19 Treatment
- PMID: 39598790
- PMCID: PMC11596935
- DOI: 10.3390/molecules29225403
Insights into SARS-CoV-2: Small-Molecule Hybrids for COVID-19 Treatment
Abstract
The advantages of a treatment modality that combines two or more therapeutic agents with different mechanisms of action encourage the study of hybrid functional compounds for pharmacological applications. Molecular hybridization, resulting from a covalent combination of two or more pharmacophore units, has emerged as a promising approach to overcome several issues and has also been explored for the design of new drugs for COVID-19 treatment. In this review, we presented an overview of small-molecule hybrids from both natural products and synthetic sources reported in the literature to date with potential antiviral anti-SARS-CoV-2 activity.
Keywords: COVID-19; SARS-CoV-2 Mpro; antiviral activity; drug design; molecular hybridization; natural product hybrids; small molecules.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

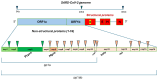
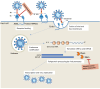
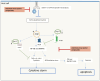
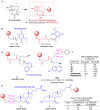
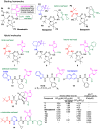
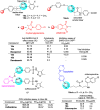
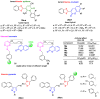
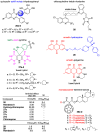
References
-
- World Health Organization . COVID-19 Epidemiological Update—17 September 2024. World Health Organization; Geneva, Switzerland: 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

