Cannabigerol (CBG): A Comprehensive Review of Its Molecular Mechanisms and Therapeutic Potential
- PMID: 39598860
- PMCID: PMC11597810
- DOI: 10.3390/molecules29225471
Cannabigerol (CBG): A Comprehensive Review of Its Molecular Mechanisms and Therapeutic Potential
Abstract
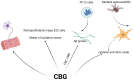
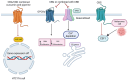
Cannabigerol (CBG), a non-psychoactive cannabinoid found in cannabis, has emerged as a promising therapeutic agent with a diverse range of potential applications. Unlike its well-known counterpart tetrahydrocannabinol (THC), CBG does not induce intoxication, making it an attractive option in the clinic. Recent research has shed light on CBG's intriguing molecular mechanisms, highlighting its potential to modulate multiple physiological processes. This review delves into the current understanding of CBG's molecular interactions and explores its therapeutic power to alleviate various conditions, including cancer, metabolic, pain, and inflammatory disorders, amongst others. We discuss how CBG interacts with the endocannabinoid system and other key signaling pathways, such as CB1, CB2, TPR channels, and α2-adrenoceptor, potentially influencing inflammation, pain, neurodegeneration, and other ailments. Additionally, we highlight the ongoing research efforts aimed at elucidating the full spectrum of CBG's therapeutic potential and its safety profile in clinical settings. Through this comprehensive analysis, we aim to provide a deeper understanding of CBG's role in promoting human health and pave the way for future research endeavors.
Keywords: Cannabigerol (CBG); cannabinoid receptor; endocannabinoid system; molecular mechanism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Anokwuru C.P., Makolo F.L., Sandasi M., Tankeu S.Y., Elisha I.L., Agoni C., Combrinck S., Viljoen A. Cannabigerol: A bibliometric overview and review of research on an important phytocannabinoid. Phytochem. Rev. 2022;21:1523–1547. doi: 10.1007/s11101-021-09794-w. - DOI
-
- Farrelly K.N., Wardell J.D., Marsden E., Scarfe M.L., Najdzionek P., Turna J., MacKillop J. The Impact of Recreational Cannabis Legalization on Cannabis Use and Associated Outcomes: A Systematic Review. Subst. Abus. Res. Treat. 2023;17:11782218231172054. doi: 10.1177/11782218231172054. - DOI - PMC - PubMed
-
- Epidiolex. [(accessed on 25 May 2024)]. Available online: https://www.epidiolex.com/
-
- Sativex®. [(accessed on 25 May 2024)]. Available online: https://www.jazzpharma.com/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

