Fast Multi-Distance Time-Domain NIRS and DCS System for Clinical Applications
- PMID: 39599151
- PMCID: PMC11598675
- DOI: 10.3390/s24227375
Fast Multi-Distance Time-Domain NIRS and DCS System for Clinical Applications
Abstract
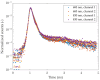
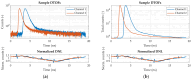
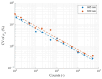
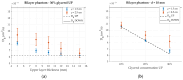
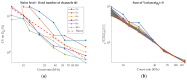
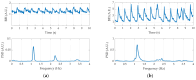
We have designed and built an improved system for combined Time-Domain Near-Infrared Spectroscopy (TD NIRS) and Diffuse Correlation Spectroscopy (DCS) measurements. The system features two independent channels, enabling TD NIRS and DCS acquisition at short and long source-detector distances to enhance depth sensitivity in layered tissues. Moreover, the device can operate at fast acquisition rates (up to 50 Hz) to monitor hemodynamic oscillations in biological tissues. An OEM (Original Equipment Manufacturer) TD NIRS device enables stable and robust acquisition of photon distribution of time-of-flight. For the DCS signals, the use of a time tagger and a software correlator allows us flexibility in post-processing. A user-friendly GUI controls TD NIRS data acquisition and online data analysis. We present results for the system characterization on calibrated tissue phantoms according to standardized protocols for performance assessment of TD NIRS and DCS devices. In-vivo measurements during rest and during vascular occlusions are also reported to validate the system in real settings.
Keywords: diffuse correlation spectroscopy; fast acquisition; hybrid device; multi-channel device; software correlator; time-domain near-infrared spectroscopy.
Conflict of interest statement
D.C. and A.T. are co-founders of PIONIRS s.r.l. (Italy). The other authors declare no conflicts of interest. The funders had no role in the design of this study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Boas D.A., Yodh A.G. Spatially Varying Dynamical Properties of Turbid Media Probed with Diffusing Temporal Light Correlation. J. Opt. Soc. Am. A. 1997;14:192–215. doi: 10.1364/JOSAA.14.000192. - DOI
-
- Amendola C., Buttafava M., Carteano T., Contini L., Cortese L., Durduran T., Frabasile L., Guadagno C.N., Karadeniz U., Lacerenza M., et al. Assessment of Power Spectral Density of Microvascular Hemodynamics in Skeletal Muscles at Very Low and Low-Frequency via near-Infrared Diffuse Optical Spectroscopies. Biomed. Opt. Express. 2023;14:5994–6015. doi: 10.1364/BOE.502618. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

