Recyclable Thermoplastic Elastomer from Furan Functionalized Hairy Nanoparticles with Polystyrene Core and Polydimethylsiloxane Hairs
- PMID: 39599208
- PMCID: PMC11598078
- DOI: 10.3390/polym16223117
Recyclable Thermoplastic Elastomer from Furan Functionalized Hairy Nanoparticles with Polystyrene Core and Polydimethylsiloxane Hairs
Abstract
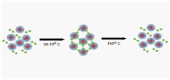
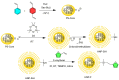
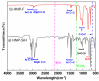
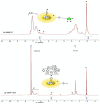
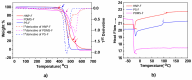
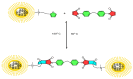
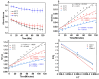
Polymers synthesized with end-of-life consideration allow for recovery and reprocessing. "Living-anionic polymerization (LAP)" and hydrosilylation reaction were utilized to synthesize hair-end furan functionalized hairy nanoparticles (HNPs) with a hard polystyrene (PS) core and soft polydimethylsiloxane (PDMS) hairs via a one-pot approach. The synthesis was carried out by first preparing the living core through crosslinking styrene with divinylbenzene using sec-butyl lithium, followed by the addition of the hexamethylcyclotrisiloxane (D3) monomer to the living core. The living polymer was terminated by dimethylchlorosilane to obtain the HNPs with Si-H functional end groups. The furan functionalization was carried out by the hydrosilylation reaction between the Si-H of the functionalized HNP and 2-vinyl furan. Additionally, furan functionalized polystyrene (PS) and polydimethylsiloxane (PDMS) were also synthesized by LAP. 1H NMR and ATR-IR spectra confirmed the successful synthesis of the target polymers. Differential scanning calorimetry showed two glass transition temperatures indicative of a polydimethylsiloxane soft phase and a polystyrene hard phase, suggesting that the HNPs are microphase separated. The furan functionalized HNPs form thermo-reversible networks upon crosslinking with bismaleimide (BMI) via a Diels-Alder coupling reaction. The kinetics of the forward Diels-Alder reaction between the functionalized polymer and BMI were studied at three different temperatures: 50 °C, 60 °C, and 70 °C by UV-Vis spectroscopy. The activation energy for the furan functionalized HNPs reaction with the bismaleimide was lower compared to the furan functionalized polystyrene and polydimethylsiloxane linear polymers. The crosslinked polymer network formed from the Diels-Alder forward reaction dissociates at around 140-154 °C, and the HNPs are recovered. The recovered HNPs can be re-crosslinked at 50 °C. The results suggest that furan functionalized HNPs are promising building blocks for preparing thermo-reversible elastomeric networks.
Keywords: Diels–Alder reaction (DA); dynamic covalent bond; glass transition temperature (Tg); kinetics; living anionic polymerization (LAP); thermoplastic elastomer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Foundation E.M. The New Plastics Economy: Rethinking the Future of Plastics. Ellen MacArthur Found; The Isle of Wight, UK: 2016. p. 120.
-
- Duflou J.R., de Moor J., Verpoest I., Dewulf W. Environmental Impact Analysis of Composite use in Car Manufacturing. CIRP Ann. 2009;58:9–12. doi: 10.1016/j.cirp.2009.03.077. - DOI
-
- Yuan C., Zhang M.Q., Rong M.Z. Application of Alkoxyamine in Self-Healing of Epoxy. J. Mater. 2014;2:6558–6566. doi: 10.1039/C4TA00130C. - DOI
-
- Toncelli C., de Reus D.C., Picchioni F., Broekhuis A.A. Properties of Reversible Diels-Alder Furan/Maleimide Polymer Networks as Function of Crosslink Density. Macromol. Chem. 2012;213:157–165. doi: 10.1002/macp.201100405. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

