Co-Optimization of Mechanical Properties and Radiopacity Through Radiopaque Filler Incorporation for Medical Tubing Applications
- PMID: 39599311
- PMCID: PMC11598453
- DOI: 10.3390/polym16223220
Co-Optimization of Mechanical Properties and Radiopacity Through Radiopaque Filler Incorporation for Medical Tubing Applications
Abstract
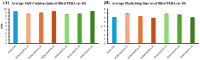
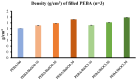
Medical tubing, particularly cardiovascular tubing, is a critical area of research where continuous improvements are necessary to advance medical devices and improve patient care. While polymers are fundamental for these applications, on their own they present several limitations such as insufficient X-ray contrasting capabilities. As such, polymer composites utilizing radiopaque fillers are a necessity for this application. For medical tubing in vivo, radiopacity is a crucial parameter that virgin polymers alone fall short in achieving due to limited X-ray absorption. To address this shortcoming, inorganic radiopaque fillers such as barium sulphate (BaSO4) and bismuth oxychloride (BiOCl) are incorporated into polymer matrices to increase the X-ray contrast of the manufactured tubing. It is also known, however, that the incorporation of these fillers can affect the mechanical, physical, and thermal properties of the finished product. This research evaluated the impact of incorporating the two aforementioned fillers into Pebax® 6333 SA01 MED at three different loading levels (10, 20, and 30 wt.%) on the physical, thermal, and mechanical properties of the composite. Composites were prepared by twin screw extrusion and injection molding followed by characterization of the mechanical (tensile, impact, and flexural), thermal (DSC), rheological (MFI), and physical (density and ash content) properties. The performed analysis shows that BiOCl enhanced the aesthetic properties, increased stiffness, and maintained flexibility while having minimal impact on the tensile and impact properties. When comparing BiOCl to BaSO4-filled composites, it was clear that depending on the application of the polymer composite, BiOCl may provide more desirable properties. The study highlights the importance of optimizing filler concentration and processing conditions to achieve desired composite properties for specific medical applications.
Keywords: PEBAX; X-ray; medical device; radiopacity; tubing.
Conflict of interest statement
Authors Alan Nugent, Maurice Kelly, and Joseph Molloy were employed by the company Innovative Polymer Compounds (IPC). The remaining authors declare that research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures







References
-
- Bitkina O.V.l., Kim H.K., Park J. Usability and user experience of medical devices: An overview of the current state, analysis methodologies, and future challenges. Int. J. Ind. Ergon. 2020;76:102932. doi: 10.1016/j.ergon.2020.102932. - DOI
-
- Biesiekierski A., Munir K., Li Y., Wen C. 2—Material selection for medical devices. In: Wen C., editor. Metallic Biomaterials Processing and Medical Device Manufacturing. Woodhead Publishing; Cambridge, UK: 2020. pp. 31–94. - DOI
-
- Newman D.K., New P.W., Heriseanu R., Petronis S., Håkansson J., Håkansson M.Å., Lee B.B. Intermittent catheterization with single- or multiple-reuse catheters: Clinical study on safety and impact on quality of life. Int. Urol. Nephrol. 2020;52:1443–1451. doi: 10.1007/s11255-020-02435-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

