The Effect of Vitamin D Supplementation Post COVID-19 Infection and Related Outcomes: A Systematic Review and Meta-Analysis
- PMID: 39599582
- PMCID: PMC11597733
- DOI: 10.3390/nu16223794
The Effect of Vitamin D Supplementation Post COVID-19 Infection and Related Outcomes: A Systematic Review and Meta-Analysis
Abstract
Background: Vitamin D's role in COVID-19 management remains controversial. This meta-analysis aimed to evaluate the efficacy of vitamin D supplementation in patients with SARS-CoV-2 infection, focusing on mortality, intensive care unit (ICU) admissions, intubation rates, and hospital length of stay (LOS).
Methods: A systematic review of PubMed/MEDLINE, Scopus, Cochrane, and Google Scholar databases was conducted. Randomized controlled trials (RCTs) and analytical studies investigating vitamin D supplementation in COVID-19 patients were included. The meta-analysis was performed using STATA MP 18.5, employing random-effect or fixed-effect models based on heterogeneity.
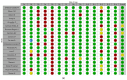
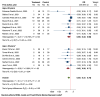
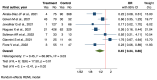
Results: Twenty-nine studies (twenty-one RCTs, eight analytical) were analyzed. Vitamin D supplementation significantly reduced ICU admissions (OR = 0.55, 95% CI: 0.37 to 0.79) in RCTs and analytical studies (OR = 0.35, 95% CI: 0.18 to 0.66). Intubation rates were significantly reduced in RCTs (OR = 0.50, 95% CI: 0.27 to 0.92). Mortality reduction was significant in analytical studies (OR = 0.45, 95% CI: 0.24 to 0.86) but not in RCTs (OR = 0.80, 95% CI: 0.61 to 1.04). Subgroup analyses revealed more pronounced effects in older patients and severe COVID-19 cases. LOS showed a non-significant reduction (mean difference = -0.62 days, 95% CI: -1.41 to 0.18).
Conclusions: This meta-analysis suggests potential benefits of vitamin D supplementation in COVID-19 patients, particularly in reducing ICU admissions. However, the evidence varies across outcomes and patient subgroups. Discrepancies between RCTs and analytical studies highlight the need for further large-scale, well-designed trials accounting for baseline vitamin D status, standardized supplementation protocols, and patient characteristics to inform clinical guidelines for vitamin D use in COVID-19 management.
Keywords: COVID-19; intensive care unit; mortality; vitamin D.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Molina P., Carrero J.J., Bover J., Chauveau P., Mazzaferro S., Torres P.U., European Renal Nutrition (ERN) and Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Working Groups of the European Renal Association-European Dialysis Transplant Association (ERA-EDTA) Vitamin D, a modulator of musculoskeletal health in chronic kidney disease. J. Cachexia Sarcopenia Muscle. 2017;8:686–701. doi: 10.1002/jcsm.12218. - DOI - PMC - PubMed
-
- Bover J., Ruiz C.E., Pilz S., Dasilva I., Díaz M.M., Guillén E. Vitamin D Receptor and Interaction with DNA: From Physiology to Chronic Kidney Disease. In: Ureña Torres P., Cozzolino M., Vervloet M., editors. Vitamin D in Chronic Kidney Disease. Springer; Cham, Switzerland: 2016. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

