Lactobacillus plantarum and Bifidobacterium longum Alleviate High-Fat Diet-Induced Obesity and Depression/Cognitive Impairment-like Behavior in Mice by Upregulating AMPK Activation and Downregulating Adipogenesis and Gut Dysbiosis
- PMID: 39599597
- PMCID: PMC11597813
- DOI: 10.3390/nu16223810
Lactobacillus plantarum and Bifidobacterium longum Alleviate High-Fat Diet-Induced Obesity and Depression/Cognitive Impairment-like Behavior in Mice by Upregulating AMPK Activation and Downregulating Adipogenesis and Gut Dysbiosis
Abstract
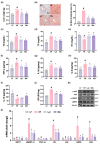
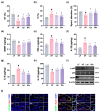
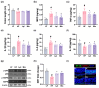
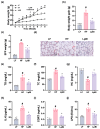
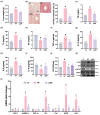
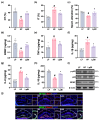
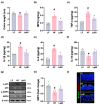
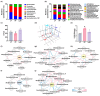
Background/Objective: Long-term intake of a high-fat diet (HFD) leads to obesity and gut dysbiosis. AMP-activated protein kinase (AMPK) is a key regulator of energy metabolism. Herein, we investigated the impacts of Lactobacillus (Lactiplantibacillus) plantarum P111 and Bifidobacterium longum P121, which suppressed dexamethasone-induced adipogenesis in 3T3 L1 cells and increased lipopolysaccharide-suppressed AMPK activation in HepG2 cells, on HFD-induced obesity, liver steatosis, gut inflammation and dysbiosis, and depression/cognitive impairment (DCi)-like behavior in mice. Methods: Obesity is induced in mice by feeding with HFD. Biomarker levels were measured using immunoblotting, enzyme-linked immunosorbent assay, and immunofluorescence staining. Results: Orally administered P111, P121, or their mix LpBl decreased HFD-induced body weight gain, epididymal fat pad weight, and triglyceride (TG), total cholesterol (TC), and lipopolysaccharide levels in the blood. Additionally, they downregulated HFD-increased NF-κB activation and TNF-α expression in the liver and colon, while HFD-decreased AMPK activation was upregulated. They also suppressed HFD-induced DCi-like behavior and hippocampal NF-κB activation, NF-κB-positive cell population, and IL-1β and TNF-α levels, while increasing the hippocampal BDNF-positive cell population and BDNF level. The combination of P111 and P122 (LpBl) also improved body weight gain, liver steatosis, and DCi-like behavior. LpBl also mitigated HFD-induced gut dysbiosis: it decreased Desulfovibrionaceae, Helicobacteriaceae, Coriobacteriaceae, and Streptococcaceae populations and lipopolysaccharide production, which were positively correlated with TNF-α expression; and increased Akkermansiaceae, Bifidobacteriaceae, and Prevotellaceae populations, which were positively correlated with the BDNF expression. Conclusions: P111 and/or P121 downregulated adipogenesis, gut dysbiosis, and NF-κB activation and upregulatde AMPK activation, leading to the alleviation of obesity, liver steatosis, and DCi.
Keywords: Bifidobacterium longum; Lactobacillus plantarum; gut microbiota; liver steatosis; obesity; psychiatric disorder.
Conflict of interest statement
Author Dong-Hyun Kim was employed by the company PBLbioLab, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

