Emerging Threats of Highly Pathogenic Avian Influenza A (H5N1) in US Dairy Cattle: Understanding Cross-Species Transmission Dynamics in Mammalian Hosts
- PMID: 39599818
- PMCID: PMC11598956
- DOI: 10.3390/v16111703
Emerging Threats of Highly Pathogenic Avian Influenza A (H5N1) in US Dairy Cattle: Understanding Cross-Species Transmission Dynamics in Mammalian Hosts
Abstract
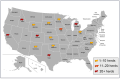
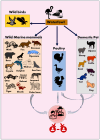
The rapid geographic spread of the highly pathogenic avian influenza (HPAI) A(H5N1) virus in poultry, wild birds, and other mammalian hosts, including humans, raises significant health concerns globally. The recent emergence of HPAI A(H5N1) in agricultural animals such as cattle and goats indicates the ability of the virus to breach unconventional host interfaces, further expanding the host range. Among the four influenza types-A, B, C, and D, cattle are most susceptible to influenza D infection and serve as a reservoir for this seven-segmented influenza virus. It is generally thought that bovines are not hosts for other types of influenza viruses, including type A. However, this long-standing viewpoint has been challenged by the recent outbreaks of HPAI A(H5N1) in dairy cows in the United States. To date, HPAI A(H5N1) has spread into fourteen states, affecting 299 dairy herds and causing clinical symptoms such as reduced appetite, fever, and a sudden drop in milk production. Infected cows can also transmit the disease through raw milk. This review article describes the current epidemiological landscape of HPAI A(H5N1) in US dairy cows and its interspecies transmission events in other mammalian hosts reported across the globe. The review also discusses the viral determinants of tropism, host range, adaptative mutations of HPAI A(H5N1) in various mammalian hosts with natural and experimental infections, and vaccination strategies. Finally, it summarizes some immediate questions that need to be addressed for a better understanding of the infection biology, transmission, and immune response of HPAI A(H5N1) in bovines.
Keywords: H5N1; cattle; high pathogenic avian influenza (HPAI); transmission.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The emergence of highly pathogenic avian influenza H5N1 in dairy cattle: implications for public health, animal health, and pandemic preparedness.Eur J Clin Microbiol Infect Dis. 2025 Aug;44(8):1817-1833. doi: 10.1007/s10096-025-05147-z. Epub 2025 May 14. Eur J Clin Microbiol Infect Dis. 2025. PMID: 40369349 Free PMC article. Review.
-
Avian influenza A (H5N1) virus in dairy cattle: origin, evolution, and cross-species transmission.mBio. 2024 Dec 11;15(12):e0254224. doi: 10.1128/mbio.02542-24. Epub 2024 Nov 13. mBio. 2024. PMID: 39535188 Free PMC article. Review.
-
Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle.Nature. 2024 Oct;634(8034):669-676. doi: 10.1038/s41586-024-07849-4. Epub 2024 Jul 25. Nature. 2024. PMID: 39053575 Free PMC article.
-
Comprehensive Insights into Highly Pathogenic Avian Influenza H5N1 in Dairy Cattle: Transmission Dynamics, Milk-Borne Risks, Public Health Implications, Biosecurity Recommendations, and One Health Strategies for Outbreak Control.Pathogens. 2025 Mar 13;14(3):278. doi: 10.3390/pathogens14030278. Pathogens. 2025. PMID: 40137763 Free PMC article. Review.
-
Emergence and interstate spread of highly pathogenic avian influenza A(H5N1) in dairy cattle in the United States.Science. 2025 Apr 25;388(6745):eadq0900. doi: 10.1126/science.adq0900. Epub 2025 Apr 25. Science. 2025. PMID: 40273240
Cited by
-
Exploring Avian Influenza Viruses in Yakutia-The Largest Breeding Habitat of Wild Migratory Birds in Northeastern Siberia.Viruses. 2025 Apr 27;17(5):632. doi: 10.3390/v17050632. Viruses. 2025. PMID: 40431644 Free PMC article.
-
The emergence of highly pathogenic avian influenza H5N1 in dairy cattle: implications for public health, animal health, and pandemic preparedness.Eur J Clin Microbiol Infect Dis. 2025 Aug;44(8):1817-1833. doi: 10.1007/s10096-025-05147-z. Epub 2025 May 14. Eur J Clin Microbiol Infect Dis. 2025. PMID: 40369349 Free PMC article. Review.
-
Highly Pathogenic Avian Influenza (H5N1) Clade 2.3.4.4b in Cattle: A Rising One Health Concern.Animals (Basel). 2025 Jul 3;15(13):1963. doi: 10.3390/ani15131963. Animals (Basel). 2025. PMID: 40646862 Free PMC article. Review.
-
Occupational Risk from Avian Influenza Viruses at Different Ecological Interfaces Between 1997 and 2019.Microorganisms. 2025 Jun 14;13(6):1391. doi: 10.3390/microorganisms13061391. Microorganisms. 2025. PMID: 40572281 Free PMC article. Review.
-
Research publications and global manufacture of veterinary vaccines against avian influenza A (2019-2023).Front Vet Sci. 2025 Mar 12;12:1394675. doi: 10.3389/fvets.2025.1394675. eCollection 2025. Front Vet Sci. 2025. PMID: 40144520 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

