Surfactant Protein A Inhibits Human Rhinovirus C Binding and Infection of Airway Epithelial Cells from Pediatric Asthma
- PMID: 39599822
- PMCID: PMC11598966
- DOI: 10.3390/v16111709
Surfactant Protein A Inhibits Human Rhinovirus C Binding and Infection of Airway Epithelial Cells from Pediatric Asthma
Abstract
Rhinovirus C (RV-C) infection can trigger asthma exacerbations in children and adults, and RV-C-induced wheezing illnesses in preschool children correlate with the development of childhood asthma. Surfactant protein A (SP-A) plays a critical role in regulating pulmonary innate immunity by binding to numerous respiratory pathogens. Mature SP-A consists of multiple isoforms that form the hetero-oligomers of SP-A1 and SP-A2, organized in 18-mers. In this report, we examined the efficacy of SP-A to antagonize RV-C infection using the wild-type (RV-C15) and reporter-expressing (RV-C15-GFP) viruses in differentiated nasal epithelial cells (NECs) from asthmatic and non-asthmatic children. We also determined the antiviral mechanism of action of SP-A on RV-C15 infection. The native SP-A was purified from alveolar proteinosis patients. The recombinant (r) SP-A1 and SP-A2 variants were expressed in FreeStyle™ 293-F cells. SP-A reduced the fluorescent focus-forming units (FFUs) after RV-C15-GFP infection of NECs by 99%. Both simultaneous and 4 h post-infection treatment with SP-A inhibited RV-C15 and RV-C15-GFP viral RNA load by 97%. In addition, the antiviral genes and chemokines (IFN-λ, IRF-7, MDA-5, and CXLC11) were not induced in the infected NECs due to the inhibition of RV-C propagation by SP-A. Furthermore, SP-A bound strongly to RV-C15 in a dose- and Ca2+-dependent manner, and this interaction inhibited RV-C15 binding to NECs. In contrast, rSP-A1 did not bind to solid-phase RV-C15, whereas the rSP-A2 variants, [A91, K223] and [P91, Q223], had strong binding affinities to RV-C15, similar to native SP-A. This study demonstrates that SP-A might have potential as an antiviral for RV infection and RV-induced asthma exacerbations.
Keywords: human rhinovirus C; innate immunity; pediatric asthma; pulmonary surfactant protein A.
Conflict of interest statement
J.G.L. and M.K. are co-founders of RaeSedo Inc, a startup company out of the University of Arizona aimed at the development of SP-A peptide-based therapeutics for the treatment of asthma. No products from RaeSedo Inc. were used in this study. The authors declare no conflicts of interest.
Figures


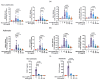

 ) or 5 mM CaCl2 (
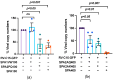
) or 5 mM CaCl2 ( ). The maximum binding (Bmax) and dissociation constant (Kd) determined by nonlinear regression analysis are indicated. (b) RV-C15 attachment to NECs was examined in the presence of various concentrations of SP-A (0 to 400 µg/mL) using cells from 4 asthmatic participants. Bound RV-C15 was quantified by RT-qPCR and results are shown as a percentage of viral binding compared to control. The concentration of SP-A that could inhibit the binding by 50% (IC50) is shown. The results represent means ± SD from 3 to 5 independent experiments.
). The maximum binding (Bmax) and dissociation constant (Kd) determined by nonlinear regression analysis are indicated. (b) RV-C15 attachment to NECs was examined in the presence of various concentrations of SP-A (0 to 400 µg/mL) using cells from 4 asthmatic participants. Bound RV-C15 was quantified by RT-qPCR and results are shown as a percentage of viral binding compared to control. The concentration of SP-A that could inhibit the binding by 50% (IC50) is shown. The results represent means ± SD from 3 to 5 independent experiments.
References
-
- Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E., Printz M.C., Lee W.M., Shult P.A., Reisdorf E. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. - DOI - PMC - PubMed
-
- Cox D.W., Khoo S.K., Zhang G., Lindsay K., Keil A.D., Knight G., Gern J.E., Laing I.A., Bizzintino J., Le Souef P.N. Rhinovirus is the most common virus and rhinovirus-C is the most common species in paediatric intensive care respiratory admissions. Eur. Respir. J. 2018;52:1800207. doi: 10.1183/13993003.00207-2018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

