Evaluation of Interfering RNA Efficacy in Treating Hepatitis B: Is It Promising?
- PMID: 39599825
- PMCID: PMC11598949
- DOI: 10.3390/v16111710
Evaluation of Interfering RNA Efficacy in Treating Hepatitis B: Is It Promising?
Abstract
Background: Despite an existing safe and effective vaccine for hepatitis B virus (HBV), it is still a major public health concern. Nowadays, several drugs are used to treat chronic hepatitis B; however, full healing remains controversial. The viral covalently closed circular DNA (cccDNA) formed by HBV forms a major challenge in its treatment, as does the ability of HBV to integrate itself into the host genome, which enables infection reactivation. Interfering RNA (RNAi) is a gene-silencing post-transcriptional mechanism which forms as a promising alternative to treat chronic hepatitis B. The aim of the present review is to assess the evolution of hepatitis B treatment approaches based on using RNA interference.
Methods: Data published between 2016 and 2023 in scientific databases (PubMed, PMC, LILACS, and Bireme) were assessed.
Results: In total, 76,949 articles were initially identified and quality-checked, and 226 eligible reports were analyzed in depth. The main genomic targets, delivery systems, and major HBV therapy innovations are discussed in this review. This review reinforces the therapeutic potential of RNAi and identifies the need for conducting further studies to fill the remaining gaps between bench and clinical practice.
Keywords: RNA interference (RNAi); chronic hepatitis; covalently closed circular DNA (cccDNA); gene therapy; hepatitis B virus (HBV).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

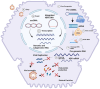
References
-
- World Health Organization (WHO) 2023, Hepatitis B. [(accessed on 18 October 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
-
- Centers for Disease Control and Prevention (CDC) [(accessed on 26 September 2024)]; Available online: https://www.cdc.gov/hepatitis/hbv/index.htm.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

