The Immune Escape Strategy of Rabies Virus and Its Pathogenicity Mechanisms
- PMID: 39599888
- PMCID: PMC11598914
- DOI: 10.3390/v16111774
The Immune Escape Strategy of Rabies Virus and Its Pathogenicity Mechanisms
Abstract
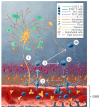
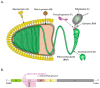
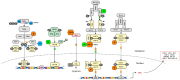
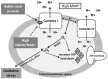
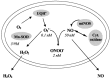
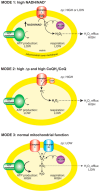
In contrast to most other rhabdoviruses, which spread by insect vectors, the rabies virus (RABV) is a very unusual member of the Rhabdoviridae family, since it has evolved to be fully adapted to warm-blooded hosts and spread directly between them. There are differences in the immune responses to laboratory-attenuated RABV and wild-type rabies virus infections. Various investigations showed that whilst laboratory-attenuated RABV elicits an innate immune response, wild-type RABV evades detection. Pathogenic RABV infection bypasses immune response by antagonizing interferon induction, which prevents downstream signal activation and impairs antiviral proteins and inflammatory cytokines production that could eliminate the virus. On the contrary, non-pathogenic RABV infection leads to immune activation and suppresses the disease. Apart from that, through recruiting leukocytes into the central nervous system (CNS) and enhancing the blood-brain barrier (BBB) permeability, which are vital factors for viral clearance and protection, cytokines/chemokines released during RABV infection play a critical role in suppressing the disease. Furthermore, early apoptosis of neural cells limit replication and spread of avirulent RABV infection, but street RABV strains infection cause delayed apoptosis that help them spread further to healthy cells and circumvent early immune exposure. Similarly, a cellular regulation mechanism called autophagy eliminates unused or damaged cytoplasmic materials and destroy microbes by delivering them to the lysosomes as part of a nonspecific immune defense mechanism. Infection with laboratory fixed RABV strains lead to complete autophagy and the viruses are eliminated. But incomplete autophagy during pathogenic RABV infection failed to destroy the viruses and might aid the virus in dodging detection by antigen-presenting cells, which could otherwise elicit adaptive immune activation. Pathogenic RABV P and M proteins, as well as high concentration of nitric oxide, which is produced during rabies virus infection, inhibits activities of mitochondrial proteins, which triggers the generation of reactive oxygen species, resulting in oxidative stress, contributing to mitochondrial malfunction and, finally, neuron process degeneration.
Keywords: BBB permeability; IFN-α/β; INOS; RABV; apoptosis; autophagy; cytkines/chemokines; immune evasion; mitochondrial dysfunction; neurons.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection.J Virol. 2014 May;88(9):4698-710. doi: 10.1128/JVI.03149-13. Epub 2014 Feb 12. J Virol. 2014. PMID: 24522913 Free PMC article.
-
T-bet Is Required for the Rapid Clearance of Attenuated Rabies Virus from Central Nervous System Tissue.J Immunol. 2015 Nov 1;195(9):4358-68. doi: 10.4049/jimmunol.1501274. Epub 2015 Sep 25. J Immunol. 2015. PMID: 26408670 Free PMC article.
-
Dual Role of Toll-Like Receptor 7 in the Pathogenesis of Rabies Virus in a Mouse Model.J Virol. 2020 Apr 16;94(9):e00111-20. doi: 10.1128/JVI.00111-20. Print 2020 Apr 16. J Virol. 2020. PMID: 32102880 Free PMC article.
-
Regulation of innate immune responses by rabies virus.Animal Model Exp Med. 2022 Oct;5(5):418-429. doi: 10.1002/ame2.12273. Epub 2022 Sep 22. Animal Model Exp Med. 2022. PMID: 36138548 Free PMC article. Review.
-
Role of chemokines in rabies pathogenesis and protection.Adv Virus Res. 2011;79:73-89. doi: 10.1016/B978-0-12-387040-7.00005-6. Adv Virus Res. 2011. PMID: 21601043 Free PMC article. Review.
Cited by
-
Lyssavirus Antibody Detection in Cave-Dwelling Bats on Cat Ba Island, Vietnam: Implications for Zoonotic Surveillance.Vet Sci. 2025 Jul 11;12(7):654. doi: 10.3390/vetsci12070654. Vet Sci. 2025. PMID: 40711314 Free PMC article.
-
Viruses and the Brain-A Relationship Prone to Trouble.Viruses. 2025 Jan 31;17(2):203. doi: 10.3390/v17020203. Viruses. 2025. PMID: 40006958 Free PMC article. Review.
-
Viral sepsis - pathophysiology and disease manifestation.Infection. 2025 Jun;53(3):775-784. doi: 10.1007/s15010-025-02486-z. Epub 2025 Feb 17. Infection. 2025. PMID: 39961996 Free PMC article. Review.
References
-
- González-Roldán J.F., Undurraga E.A., Meltzer M.I., Atkins C., Vargas-Pino F., Gutiérrez-Cedillo V., Hernández-Pérez J.R. Cost-effectiveness of the national dog rabies prevention and control program in Mexico, 1990–2015. PLoS Neglected Trop. Dis. 2021;15:e0009130. doi: 10.1371/journal.pntd.0009130. - DOI - PMC - PubMed
-
- Gunesekara A., Changalucha J., Nguyen H., Li A., Shiferaw M., Wallace R., Blanton J., Knopf L., Hyde T., Siddiqi U., et al. Overview of rabies post-exposure prophylaxis access, procurement and distribution in selected countries in Asia and Africa, 2017–2018. Vaccine. 2019;37:A6–A13. doi: 10.1016/j.vaccine.2019.04.024. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

