Single-Nucleus and Spatial Transcriptomics Revealing Host Response Differences Triggered by Mutated Virus in Severe Dengue
- PMID: 39599894
- PMCID: PMC11599075
- DOI: 10.3390/v16111779
Single-Nucleus and Spatial Transcriptomics Revealing Host Response Differences Triggered by Mutated Virus in Severe Dengue
Abstract
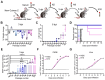
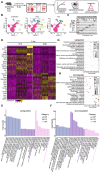
Dengue virus (DENV) infection causes various disease manifestations ranging from an asymptomatic state to severe, life-threatening dengue. Despite intensive research, the molecular mechanisms underlying the abnormal host responses and severe disease symptoms caused by evolved DENV strains is not fully understood. First, the spatial structure of mutant DENV was compared via in silico molecular modeling analysis. Second, employing single-nucleus and spatial RNA sequencing, we analyzed and verified transcriptome samples in uninfected, mild (NGC group), and severe (N10 group) liver tissues from murine models. In this study, we obtained a cumulatively mutated DENV-2 N10 with enhanced capability of replication and pathogenicity post 10 serial passages in Ifnra-/- mice. This variant caused severe damage in the liver, as compared with other organs. Furthermore, mutated DENV infection elicited stronger responses in hepatocytes. The critical host factor Nrg4 was identified. It dominated mainly via the activation of the NRG/ErbB pathway in mice with severe symptoms. We report on evolved N10 viruses with changes observed in different organisms and tissue. This evolutionary variant results in high replicability, severe pathogenicity, and strong responses in murine. Moreover, the host responses may play a role by activating the NRG/ErbB signaling pathway. Our findings provide a realistic framework for defining disturbed host responses at the animal model level that might be one of the main causes of severe dengue and the potential application value.
Keywords: Nrg4; cumulative mutation; dengue virus; severe dengue; single-nucleus RNA sequencing; spatial transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Antibody-Dependent Dengue Virus Entry Modulates Cell Intrinsic Responses for Enhanced Infection.mSphere. 2019 Sep 18;4(5):e00528-19. doi: 10.1128/mSphere.00528-19. mSphere. 2019. PMID: 31533998 Free PMC article.
-
Liver transcriptomics reveals features of the host response in a mouse model of dengue virus infection.Front Immunol. 2022 Aug 26;13:892469. doi: 10.3389/fimmu.2022.892469. eCollection 2022. Front Immunol. 2022. PMID: 36091000 Free PMC article.
-
Pathogenicity and transcriptomic resolution in dengue virus serotype 1 infected AGB6 mouse model.J Med Virol. 2024 Sep;96(9):e29895. doi: 10.1002/jmv.29895. J Med Virol. 2024. PMID: 39228306
-
Progress towards understanding the pathogenesis of dengue hemorrhagic fever.Virol Sin. 2017 Feb;32(1):16-22. doi: 10.1007/s12250-016-3855-9. Epub 2016 Nov 14. Virol Sin. 2017. PMID: 27853992 Free PMC article. Review.
-
Dengue virus pathogenesis and host molecular machineries.J Biomed Sci. 2024 Apr 22;31(1):43. doi: 10.1186/s12929-024-01030-9. J Biomed Sci. 2024. PMID: 38649998 Free PMC article. Review.
References
Publication types
MeSH terms
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

