Single-Cell Transcriptomics of Human Tonsils Reveals Nicotine Enhances HIV-1-Induced NLRP3 Inflammasome and Mitochondrial Activation
- PMID: 39599911
- PMCID: PMC11598941
- DOI: 10.3390/v16111797
Single-Cell Transcriptomics of Human Tonsils Reveals Nicotine Enhances HIV-1-Induced NLRP3 Inflammasome and Mitochondrial Activation
Abstract
Background: HIV-1 infection, even with effective antiretroviral therapy (ART), is associated with chronic inflammation and immune dysfunction, contributing to long-term health complications. Nicotine use, prevalent among people with HIV (PWH), is known to exacerbate immune activation and disease progression, but the precise biological mechanisms remain to be fully understood. This study sought to uncover the synergistic effects of HIV-1 infection and nicotine on immune cell function, focusing on beneficial insights into NLRP3 inflammasome activation, oxidative stress, and mitochondrial pathways.
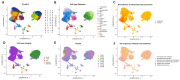
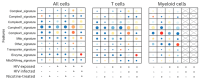
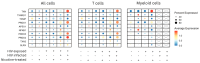
Methods: Human tonsil explants were infected with HIV-1 and exposed to nicotine. Single-cell RNA sequencing was used to profile immune cell populations and gene expression linked to inflammasome activation, oxidative stress, and mitochondrial function. Gene set enrichment analysis (GSEA) and synergy assessments were conducted to investigate how nicotine modulates immune responses in the context of HIV.
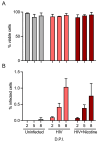
Results: The combination of HIV infection and nicotine exposure significantly increased NLRP3 inflammasome activation, thioredoxin, and components of oxidative phosphorylation.
Conclusions: This study highlights how the combined effects of HIV-1 and nicotine offer valuable insights into immune modulation, opening doors for future therapeutic strategies. Targeting the NLRP3 inflammasome and addressing nicotine use may contribute to improved outcomes for PWH.
Keywords: HIV-1; NLRP3 inflammasome; mitochondrial dysfunction; nicotine; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

