Toward Polymeric Room Temperature Acid Generators
- PMID: 39599963
- PMCID: PMC11891450
- DOI: 10.1002/open.202400289
Toward Polymeric Room Temperature Acid Generators
Abstract
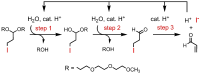
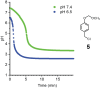
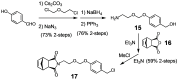
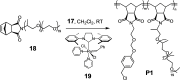
Although a variety of acid-generating molecules have been developed, the formation of toxic byproducts and the need for light-activation or temperatures that may be incompatible with physiological conditions leave room for the optimization of biocompatible acid-generators. Herein, we report 4-hydroxybenzyl chloride derivatives that generate hydrochloric acid via hydrolysis at the benzylic position at room temperature in the absence of light. Utilizing the acetal protected 4-hydroxybenzyl chloride scaffold, we access a myriad of compounds that generate acid at different rates.
Keywords: Acid amplification; Acid generator; Cancer; Drug delivery; Extra cellular pH.
© 2024 The Authors. ChemistryOpen published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

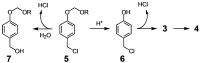
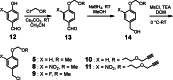
References
-
- Zivic N., Kuroishi P. K., Dumur F., Gigmes D., Dove A. P., Sardon H., Angew. Chem. Int. Ed. 2019, 58, 10410–10422. - PubMed
-
- Prajwal B., Blackwell J. M., Theofanis P., Escobedo F. A., Chem. Mater. 2023, 35, 9050–9063.
-
- Ichimura K., Chem. Rec. 2002, 2, 46–55. - PubMed
-
- Arimitsu K., Yonekura M., Furutani M., RSC Adv. 2015, 5, 80311–80317.
Grants and funding
LinkOut - more resources
Full Text Sources

