Dodecanedioic acid prevents and reverses metabolic-associated liver disease and obesity and ameliorates liver fibrosis in a rodent model of diet-induced obesity
- PMID: 39600104
- PMCID: PMC11599784
- DOI: 10.1096/fj.202402108R
Dodecanedioic acid prevents and reverses metabolic-associated liver disease and obesity and ameliorates liver fibrosis in a rodent model of diet-induced obesity
Abstract
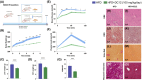
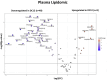
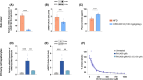
Dodecanedioic acid (DC12) is a dicarboxylic acid present in protective polymers of fruit and leaves. We explored the effects of DC12 on metabolic dysfunction-associated steatohepatitis (MASH) and obesity. DC12 supplementation (100 mg/kg/day) was added to a high-fat diet (HFD) for 8 weeks in rodents to assess its impact on obesity and MASH prevention. Rats given DC12 experienced significant reductions of weight gain, liver and visceral fat weight, and improved glucose tolerance and insulin sensitivity. Liver histology showed protection against diet-induced MASH, with reduced steatosis, hepatocyte ballooning, and fibrosis. For weight-loss and MASH reversion, rats were fed HFD for 14 weeks, followed by 6 weeks with or without DC12. DC12 supplementation (100 mg/kg/day) led to a significant reduction of weight gain and liver weight. DC12 induced white adipose tissue beiging and reduced adiposity with a decrease of visceral fat. It also improved glucose tolerance, insulin sensitivity, and reduced hepatic gluconeogenic gene expression. Liver histology revealed a significant reduction in steatosis, hepatocyte ballooning, and inflammation as well as fibrosis, indicating MASH reversal. DC12 reduced hepatic lipogenesis enzymes as well as de novo lipogenesis measured by deuterated water and increased fatty acid β-oxidation. Plasma lipid profile showed lower triglycerides and phosphatidylcholines in the DC12 group. Notably, DC12 decreased mINDY expression, the cell membrane Na+-coupled citrate transporter, reducing citrate uptake and de-novo lipogenesis, linking its effects to improved lipid metabolism and reduced steatosis. We found that during the hepatic first pass, half of the DC12 ingested with water was taken up by the liver. The concentration of DC12 in the portal vein falls within the range identified in vitro as sufficient to inhibit citrate transport in hepatocytes.
Keywords: citrate; de novo lipogenesis; dodecanedioic acid (DC12); glucose tolerance; metabolic dysfunction‐associated steatohepatitis (MASH); obesity.
© 2024 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
GA reports consulting fees from Metadeq and GHP Scientific. GM reports consulting fees from Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Medtronic, Fractyl Inc., Recor Inc. She is also Scientific Advisor of Keyron Ltd., Metadeq Inc., GHP Scientific Ltd., and Jemyll Ltd. All other authors declare no competing interests.
Figures


References
-
- Bertuzzi A, Mingrone G, Gandolfi A, Greco AV, Salinari S. Disposition of dodecanedioic acid in humans. J Pharmacol Exp Ther. 2000;292:846‐852. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

