Organic NMR crystallography: enabling progress for applications to pharmaceuticals and plant cell walls
- PMID: 39600178
- PMCID: PMC11599944
- DOI: 10.1039/d4fd00088a
Organic NMR crystallography: enabling progress for applications to pharmaceuticals and plant cell walls
Abstract
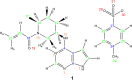
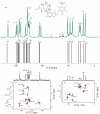
The application of NMR crystallography to organic molecules is exemplified by two case studies. For the tosylate salt of the active pharmaceutical ingredient, Ritlectinib, solid-state NMR spectra are presented at a 1H Larmor frequency of 1 GHz and a magic-angle spinning (MAS) frequency of 60 kHz. Specifically, 14N-1H heteronuclear multiple-quantum coherence (HMQC) and 1H-1H double-quantum (DQ) single-quantum (SQ) correlation experiments are powerful probes of hydrogen bonding interactions. A full assignment of the 1H, 13C and 14N/15N chemical shifts is achieved using also 1H-13C cross polarization (CP) HETCOR spectra together with gauge-including projector augmented wave (GIPAW) DFT calculation for the geometry-optimised X-ray diffraction crystal structure that is reported here (CCDC 2352028). In addition, GIPAW calculations are presented for the 13C chemical shifts in the two polymorphs of cellulose for which diffraction structures are available. For both case studies, a focus is on the discrepancy between experiment and GIPAW calculation.
Conflict of interest statement
Some of the authors are employees of Pfizer, and compound 1 is a Pfizer pharmaceutical.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

