Biologics in allergology and clinical immunology: Update on therapies for atopic diseases, urticaria, and angioedema and on safety aspects focusing on hypersensitivity reactions
- PMID: 39600395
- PMCID: PMC11590746
- DOI: 10.5414/ALX02533E
Biologics in allergology and clinical immunology: Update on therapies for atopic diseases, urticaria, and angioedema and on safety aspects focusing on hypersensitivity reactions
Abstract
The development of targeted therapies for atopic diseases, urticaria, and angioedema with biologics is progressing rapidly: New "targets" of clinical-therapeutic relevance have been identified, the corresponding targeted antibodies developed, tested in clinical trials, and approved for therapy. These include the anti-IgE antibody omalizumab (also effective and approved for the treatment of urticaria), the anti-IL-4/13 receptor-specific antibody dupilumab, the two anti-IL-13 antibodies lebrikizumab and tralokinumab, the anti-TSLP antibody tezepelumab, the two anti-IL-5 antibodies mepolizumab and reslizumab, and the anti-IL5 receptor-specific antibody benralizumab for the treatment of atopic diseases. For the treatment of hereditary angioedema, C1 inhibitor and the antibody lanadelumab (directed against kallikrein) have also long been approved as biologics in addition to low-molecular substances. Other therapeutic antibodies are in various stages of development. Furthermore, the range of indications for some very effective biologics has been successfully expanded to include additional diseases. In this context, the first results on biologic therapy of food allergy and eosinophilic esophagitis are interesting. Biologics that address different target structures are also increasingly being administered in combination, either simultaneously or sequentially, in order to achieve optimal efficacy. A developing area is the use of biologics in children and the observation of immunological and non-immunological side effects. In some cases, new unexpected side effects and hypersensitivity reactions have emerged, which in turn raise pathomechanistic questions, such as conjunctivitis with dupilumab therapy, which only appears to occur in the treatment of atopic dermatitis but not in the treatment of other atopic diseases. In dermatology, paradoxical reactions have been described under therapy with some biologics. And immune reactions of type alpha to epsilon to biologics (hypersensitivity reactions) continue to be a clinically relevant problem, whereby the selection of an alternative therapeutic agent is a challenge and the diagnostics that support this have not yet been sufficiently incorporated into routine work.
Keywords: COVID 19; anti-drug antibodies; biological allergy; children; eosinophilic oesophagitis (EoE); food allergy; hypersensitivity reactions; off-label use; pregnancy; vaccination.
© Dustri-Verlag Dr. K. Feistle.
Conflict of interest statement
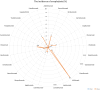
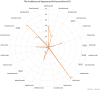
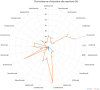
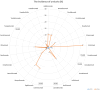
CT, AG, VF, KCB, and FB have no conflict of interest. UJ received hotel accommodation and meals for a lecture and for leading a workshop organized by ALK Abello. The fee went to her organization, the RCB. In addition, another hotel night and dinner were recently provided by ALK Abello. Her research on molecular allergology is funded by the Federal Ministry of Education and Science, the Federal Ministry of Food and Agriculture (BMEL), the German Research Foundation and the Kanert Foundation: all outside the topic of this article. The Federal Ministry of Technology, Economics and Technology has funded their research on assays for the detection of anti-drug antibodies via the AiF-ZIM program. ST reports support for consultancy, lectures, and other scientific activities from AbbVie, Janssen/ JNJ, Leo Pharma, Lilly, Novartis, and Regeneron/Sanofi outside the submitted work, memberships: DGAKI, DDG, EAACI. RT received research support from Sanofi-Genzyme and the Hautnetz Leipzig/Westsachsen e.V. as well as fees for lectures and consultations from ALK-Abello, Takeda, Novartis, Sanofi-Genzyme, Abbvie, and support for congress visits from Takeda. LK reports grants and/or personal fees from Allergopharma, MEDA / Mylan, HAL Allergie, ALK Abelló, LETI Pharma, Stallergenes, Quintiles, Sanofi, ASIT Biotech, Lofarma, Allergy Therapeut., AstraZeneca, GSK, Inmunotk and Cassela med outside the submitted work; and memberships: AeDA, DGHNO, German Academy of Allergology and Clinical Immunology, HNO-BV, GPA, EAACI. MWo reports support for consultancies, lectures and other scientific activities from ALK-Abelló Arzneimittel GmbH, Abbvie, Eli Lilly, Mylan Germany GmbH, Bencard Allergie GmbH, Novartis AG, Biotest AG, Sanofi-Aventis Deutschland GmbH, HAL Allergie GmbH, DBV Technologies S.A, Aimmune Therapeutics UK Limited, Regeneron Pharmaceuticals, Inc, Stallergenes GmbH. S. Seurig reports support for consultations, lectures, and other scientific activities by Allergopharma, ALK-Abelló Arzneimittel GmbH, AstraZeneca, Takeda outside the submitted work, memberships: DGAKI, DGP, EAACI TZ reports support for consultations, lectures and other scientific activities by AstraZeneca, AbbVie, ALK, Almirall, Astellas, Bayer Health Care, Bencard, Berlin Chemie, FAES, HAL, Henkel, Kryolan, Leti, Lofarma, L’Oreal, Meda, Menarini, Merck, MSD, Novartis, Pfizer, Sanofi, Sanoflore, Stallergenes, Takeda, Teva, UCB as well as responsible participation in the following organizations: Committee member, WHO initiative “Allergic Rhinitis and its Impact on Asthma” (ARIA), Member of the Board, German Society for Allergy and Clinical Immunology (DGAKI), Head, European Centre for Allergy Research Foundation (ECARF), Secretary General, Global Allergy and Asthma European Network (GA2LEN), Member, Committee on Allergy Diagnosis and Molecular Allergology, World Allergy Organization (WAO). TW reports support for consultancy, lectures, and other scientific activities from AbbVie, ALK Abello, Almirall, Astellas, Bencard, Galderma, Janssen/JNJ, Leo Pharma, Leti, Lilly, Novartis, Pfizer, Regeneron/Sanofi, Stallergen. MWa has received fees for consulting, lectures or research support from the following companies in the past 3 years: Allergopharma, ALK-Abelló, AstraZeneca, CSL Behring, Genzyme, GSK, HAL Allergie, Infectopharm, LETI Pharma, Novartis, Regeneron, Sanofi, Stallergenes, Takeda. HR: Co-Founder STERNA Biologicals and SECARNA Pharmaceuticals. Table 1.Frequency of hypersensitivity reaction to biologics. BiologicTargetAuthorYearHSR %IR %ISR %Urticaria %Anaphylaxis %AdalimumabTNF-αPuxeddu et al. [243]20123.5–1.51.50Tarkiainen et al. [244]201518.117.0––EMA [245]20231.0 – 10.012.91.0 – 10.00.01 – 0.1FDA [246]20236.05.0 – 20.06.0–BenralizumabIL-5RCastro et al. [247]2014–16.0––Park et al. [248]2019–00 – 2.0–Liu et al. [249]2019–2.6–17.5––FDA [250]20193.02.23.03.0Bourdin et al. [251]20190 – 3.23.2 – 6.5––EMA [252]2023up to 10.02.2up to 10.0–Yamaguchi et al. [253]20240.3––0.3BrodalumabIL-17RAFDA [254]2017–1.51.0–Iznardo et al. [255]2020< 1.01.8––Kim et al. [256]2023–1.3––EMA [257]2023–1.0 – 10.0–0.01 – 0.1CertolizumabTNFαFDA [258]2022–1.7 – 3.2––EMA [259]2023–1.0 – 10.0–0.01 – 0.1Kim et al. [256]2023–3.9––DupilumabIL-4RαOu et al. [260]2018–13.2––Halling et al [261]2021–5.3––EMA [262]2023–1.0 – 10.0–0.01 – 0.1Kim et al. [256]2023–11.3––FDA [263]2024< 1.06.0 – 38.0< 1.0< 1.0Simpson et al. [264]2024–3.0––Yew et al. [265]2024–2.05.9–EtanerceptTNF-α-RIIPuxeddu et. al. [243]20125.3–1.62.00.8Tarkiainen et al. [244]201511.37.5––Girolomoni et al. [266]2018–10 – 49.0––Codreanu et al. [267]2019–0.8–0.8FDA [268]2023< 1.015 – 43.0< 2.0< 2.0EMA [269]2024–13.6 – 36.00.1 – 1.00.01 – 0.1EtokimabIL-33Chen et al. [270]2019––25.016.7–Chinthrajah et al. [271]2019––26.76.70NCT03614923 - Eclipse [272]2022––2.8 – 5.7––FezakinumabIL-22–––––––GaradacimabFXIIaCraigh et al. [273]20230–5.0–0GuselkumabIL-23Langley et al. [274]201801.10FDA [275]2020–4.5< 1.0–European Comission [276]20200.1 – 1.01.0 – 10.00.1 – 1.00.1 – 1.0Coates et al. [277]2021–1.80.40McInnes et al. [278]2022–2.5 – 2.7-0Danese et al. [279]202404.0-0InfliximabTNF-αMaggi et al. [280]2011–1.0 – 27.0–––Puxeddu et al. [243]201213.8–04.49.3Tarkiainen et al. [244]201534.1–1.9–1.9Lichtenstein et al. [281]2015–5.0 – 23.0–4.0–Panés et al. [282]2019–13.0–––FDA [283]2023–< 20.0–< 1.0< 1.0EMA [284]2024–> 10.01.0 – 10.01.0 – 10.00.1 – 1.0IxekizumabIL-17AFDA [285]2022≤ 0.117.0≤ 0.1≤ 0.1EMA [286]2023–> 10.00.1 – 1.00.01 – 0.1Kim et al. [256]2023–11.2––Mastorino et al. [287]2023–3.1––Ying et al. [288]20230.33.0 – 9.73.3–LanadelumabPlasma kallikreinFDA [289]20181.045.0 – 57.0––Craig et al. [290]2021–5.1 – 62.5––Hide et al. [291]2023–50.0––EMA [292]20241.252.4––LebrikizumabIL-13Hanania et al. [293]20150 – 0.911.1 – 20.5–0 – 0.9Hanania et al. [294]2016–6.0 – 10.0–< 1.0Simpson et al. [295]2018–1.3–0Korenblat et al. [296]2018–2.9–1.0Austin [297]202007.0–0EMA [289]2023–2.6––Paller et al. [299]2023–2.42.90Stein Gold et al. [300]202302.6–0LigelizumabCε3 domain of IgEGauvreau et al. [301]2016–12.5 – 25.000Maurer et al. [302]2019–4.0 – 7.0–0Wood et al. [239]2022––17.00.4Maurer et al. [303]20246.0 – 11.04.0 – 11.0–< 1.0MepolizumabIL-5Pavord et al. [304]2012≤ 1.05.0–12.0––0Lugogo et al. [305]2016< 1.0<1.03.0–0Leung et al. [306]20170 – 1.05.0 – 12.03.0 – 9.04.0 – 16.00.002Khatri et al. [307]20192.0–12.0–0Chapman et al. [308]2019< 1.0–3.0< 1.00EMA [309]20221.9 – 3.0–6.0 – 7.0–0FDA [310]20231.0 – 4.02.0 – 15.0––Ishii et al. [311]2023< 1.0––< 1.0NemolizumabIL-31RαNemoto et al. [312]2016–––0Kabashima et al. [313]2018–2.02.0 – 6.0–Silverberg et al. [314]2020–1.8 – 3.5––Ständer et al. [315]2020–3.0––Kabashima et al. [185]2022–< 1.0––Igarashi et al. [316]2023–2.2––OmalizumabIgECox et al. [317]2007< 0.2––0.09Di Bona et al. [318]2017–3.41.00FDA [319]a2023–12.0 – 45.00.20.1FDA [319]b2023–0.6 – 2.7––EMA [320]2023–2.70.1–1.00.2Kim et al. [256]2023–4.5––ReslizumabIL-5Castro et al. [321]2015––1.0 – 2.0–< 1.0Murphy et al. [322]2017< 1.0< 1.0< 1.0< 1.00FDA [323]2019––––0.3Virschow et al. [324]2020––––< 1.0Bernstein et al. [325]20200–6.0 – 11.0––EMA [326]20230.190.19––0.19RituximabCD20Terrier et al. [327]2010–9.0––1.4Maggi et al. [280]2011–10 – 77.0–––FDA (s.c.) [328]2021––13–26.0––FDA (i.v.) [329]2021–≥ 25.0–2.0 – 8.0< 2.0BCCA [330]20241.0 – 10.014 – 77.020.07.0–EMA [331]20241.0 – 10.0> 10.0< 20.01.0 – 10.00.01 – 0.1Riveiro-Barciela et al. [332]2024–9.0––2.8SecukinumabIL-17ABlauvelt [333]2016–0.7––Deodhar et al. [334]20192.40.8–1.3––Grace et al. [335]2020–25.0––Asawanonda et al. [336]2022–0.6––Li et al. [337]2022–2.3––FDA [338]20230.01 – 0.1–0.6 – 1.2–EMA [339]2023––0.1 – 1.00.01 – 0.1Kim et al. [256]2023–1.9––TezepelumabAnti-TSLPMenzies-Gow et al. [340]2021–3.6–0Corren et al. [341]2023–4.0–0EMA [342]2024–3.8––TralokinumabIL-13Wollenberg et al. [343]2019–5.2––Panettieri et al. [344]2018–4.0–5.4–0Busse et al. [345]2019–15.7–0Carlsson et al. [346]201913.2 – 25.9–< 1.00Silverberg et al. [347]20216.7FDA [348]2023–7.4 – 11.1––EMA [349]2023–7.2––Paller et al. [40]2023–2.1 – 9.2–0UpadacitinibJAK inhibitorFDA [350]20232.0 – 3.0–2.0 – 3.02.0 – 3.0EMA [351]20230.1 – 1.01.0 – 10.00.1 – 1.0UstekinumabIL-12 / IL-23Ghosh et al. [352]2019<1.00.1–< 1.00FDA [353]20230.08–1.0 – 5.0–0.1EMA [354]20230.1 – 1.01.90.1 – 10.00.080.01 – 0.1Kim et al. [256]2023––2.80.1 – 1.0– aResults of clinical studies with asthma in FDA 2023 label. bResults of pooled chronic idiopathic urticaria trials in FDA 2023 label. JAK = Janus kinase; TSLP = thymic stromal lymphopoietin; HSR = hypersensitivity reaction; IR = infusion reaction, substance-specific; ISR = injection-site reaction. Figure 1The incidence of anaphylaxis (%).Figure 2The incidence of hypersensitivity reactions (%).Figure 3The incidence of injection-site reactions (%). Figure 4The incidence of urticaria (%). Table 2.Laboratory tests before administration of immunosuppressive or immunomodulating drugs [1]. Infectious agentsTestHepatitis B virus– anti-HBS quantified – HBs Antigen, anti-HBs and – anti-HBc Hepatitis C virus(anti-Hepatitis C)Hepatitis A virus(anti-HAV IgG)Epstein-Barr virusanti-EBVCytomegalovirusanti-CMV IgG and IgMHerpes virusanti-HSV q and 2: IgG and IgMVaricella zoster virusAnti-VZ IgGSyphilis VDRL or TPPA Table 4.Biologics investigated in ongoing studies for the indication of eosinophilic esophagitis. NameTargetPhaseBarzolvolimabKIT-TKIPhase II [228]EtrasimodSphingosine 1P-R modulatorPhase II [229]CendakimabIL-13 inhibitorPhase III [230]TezepelumabAnti-TSLP antibodyPhase III [231] Table 3.Biologics investigated in ongoing studies for the indication of chronic spontaneous urticaria.NameTargetPhaseUB-221IgER antagonistsPhase II [221]LirentelimabSiglec-8 agonistPhase II [222]BarzolvolimabKIT-TKIPhase II [223]RilzabrutinibBruton-TKIPhase II [224]TAS-5315Bruton-TKIPhase II [225]
Figures
References
-
- Jappe U Bergmann K Gülsen A Klimek L Plilipp S Pickert J Rauber-Ellinghaus M Renz H Taube C Treudler R Wagenmann M Werfel T Worm M Zuberbier T Biologika bei atopischen Erkrankungen: Indikationsstellung, Nebenwirkungsmanagement und neue Entwicklungen. Allergologie. 2021; 41: 54–80.
-
- van Buul AR Taube C Treatment of severe asthma: entering the era of targeted therapy. Expert Opin Biol Ther. 2015; 15: 1713–1725. - PubMed
-
- Hekking PW Wener RR Amelink M Zwinderman AH Bouvy ML Bel EH The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015; 135: 896–902. - PubMed
-
- Haasler I Taube C Biologicals in the treatment of bronchial asthma. Pneumologie. 2017; 71: 684–698. - PubMed
Publication types
LinkOut - more resources
Full Text Sources