6,6'-Biindeno[1,2- b]fluorene: an open-shell indenofluorene dimer
- PMID: 39600505
- PMCID: PMC11587529
- DOI: 10.1039/d4sc03996c
6,6'-Biindeno[1,2- b]fluorene: an open-shell indenofluorene dimer
Abstract
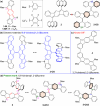
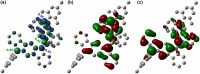
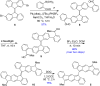
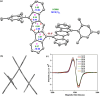
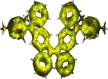
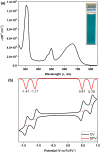
Nakano et al. reported that the antiaromatic indenofluorene (IF) isomers are diradicaloid molecules having varying degrees of open-shell character, with indeno[1,2-b]fluorene displaying a weaker diradical character index (y 0 = 0.072). Unlike 6,12-trimethylsilylethynyl disubstituted [1,2-b]IF, the 6,12-aryl disubstituted [1,2-b]IF derivatives did not show any experimental evidence of diradical properties. This raised the question of whether a [1,2-b]IF dimer would prefer a closed-shell or an open-shell ground state. To address this, herein we report the synthesis of a 6,6'-biindeno[1,2-b]fluorene derivative, which is a [1,2-b]IF dimer, constructed by linking two [1,2-b]IF units with a C-C single bond at carbons 6 and 6' bearing the largest orbital coefficients for the highest occupied and lowest unoccupied molecular orbitals (HOMO and LUMO). The C6-C6' linkage effectively narrowed the HOMO-LUMO gap while the strong desire to avoid s-indacene antiaromaticity restored two Clar sextets in two proaromatic para-quinodimethane subunits, resulting in an open-shell bifluorenylidene-type diradicaloid (y 0 = 0.268) ground state with minor tetraradical character index (y 1 = 0.007). The open-shell nature was confirmed by single crystal X-ray and electron paramagnetic resonance analyses, and supported by theoretical calculations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Wu J., Diradicaloids, Jenny Stanford Publishing, New York, 2022
-
- Hu J. Xiang Q. Tian X. Ye L. Wang Y. Ni Y. Chen X. Liu Y. Chen G. Sun Z. J. Am. Chem. Soc. 2024;146:10321–10330. doi: 10.1021/jacs.3c11585. - DOI - PubMed
- Kuriakose F. Commodore M. Hu C. Fabiano C. J. Sen D. Li R. R. Bisht S. Üngör Ö. Lin X. Strouse G. F. DePrince III A. E. Lazenby R. A. Mentink-Vigier F. Shatruk M. Alabugin I. V. J. Am. Chem. Soc. 2022;144:23448–23464. doi: 10.1021/jacs.2c09637. - DOI - PubMed
- Borissov A. Chmielewski P. J. Gómez García C. J. Lis T. Stępień M. Angew. Chem., Int. Ed. 2023;62:e202309238. doi: 10.1002/anie.202309238. - DOI - PubMed
- Ravat P. Šolomek T. Rickhaus M. Häussinger D. Neuburger M. Baumgarten M. Juríček M. Angew. Chem., Int. Ed. 2016;55:1183–1186. doi: 10.1002/anie.201507961. - DOI - PubMed
- Feofanov M. Akhmetov V. Sharapa D. I. Amsharov K. Org. Lett. 2020;22:5741–5745. doi: 10.1021/acs.orglett.0c01717. - DOI - PubMed
-
- Muhammad S. Nakano M. Al-Sehemi A. G. Kitagawa Y. Irfan A. Chaudhry A. R. Kishi R. Ito S. Yoneda K. Fukuda K. Nanoscale. 2016;8:17998–18020. doi: 10.1039/C6NR06097H. - DOI - PubMed
- Nakano M. Chem. Rec. 2017;17:27–62. doi: 10.1002/tcr.201600094. - DOI - PubMed
- Zhou Z. Yang K. He L. Wang W. Lai W. Yang Y. Dong Y. Xie S. Yuan L. Zeng Z. J. Am. Chem. Soc. 2024;146:6763–6772. doi: 10.1021/jacs.3c13270. - DOI - PubMed
- Nakano M. Kishi R. Nitta T. Kubo T. Nakasuji K. Kamada K. Ohta K. Champagne B. Botek E. Yamaguchi K. J. Phys. Chem. A. 2005;109:885–891. doi: 10.1021/jp046322x. - DOI - PubMed
- Minami T. Nakano M. J. Phys. Chem. Lett. 2012;3:145–150. doi: 10.1021/jz2015346. - DOI - PubMed
-
- Liu C. Sandoval-Salinas M. E. Hong Y. Gopalakrishna T. Y. Phan H. Aratani N. Herng T. S. Ding J. Yamada H. Kim D. Casanova D. Wu J. Chem. 2018;4:1586–1595. doi: 10.1016/j.chempr.2018.03.020. - DOI
- Das S. Herng T. S. Zafra J. L. Burrezo P. M. Kitano M. Ishida M. Gopalakrishna T. Y. Hu P. Osuka A. Casado J. Ding J. Casanova D. Wu J. J. Am. Chem. Soc. 2016;138:7782–7790. doi: 10.1021/jacs.6b04539. - DOI - PubMed
- Horii K. Kishi R. Nakano M. Shiomi D. Sato K. Takui T. Konishi A. Yasuda M. J. Am. Chem. Soc. 2022;144:3370–3375. doi: 10.1021/jacs.2c00476. - DOI - PubMed
-
- Jousselin-Oba T. Mamada M. Marrot J. Maignan A. Adachi C. Yassar A. Frigoli M. J. Am. Chem. Soc. 2019;141:9373–9381. doi: 10.1021/jacs.9b03488. - DOI - PubMed
- Zong C. Zhu X. Xu Z. Zhang L. Xu J. Guo J. Xiang Q. Zeng Z. Hu W. Wu J. Li R. Sun Z. Angew. Chem., Int. Ed. 2021;60:16230–16236. doi: 10.1002/anie.202105872. - DOI - PubMed
- Hu X. Wang W. Wang D. Zheng Y. J. Mater. Chem. C. 2018;6:11232–11242. doi: 10.1039/C8TC04484H. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

