Reactivity of metal hydrides with CO2: going beyond formate with a high-valent cationic pentahydride Mo(vi) complex
- PMID: 39600508
- PMCID: PMC11587530
- DOI: 10.1039/d4sc04345f
Reactivity of metal hydrides with CO2: going beyond formate with a high-valent cationic pentahydride Mo(vi) complex
Abstract
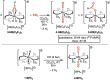
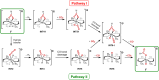
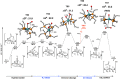
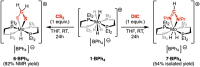
The cationic molybdenum pentahydride complex [MoH5(depe)2]+ (depe = 1,2-bis(diethylphosphino)ethane) is shown to undergo two consecutive reactions with carbon dioxide. In the initial, room-temperature process, classical insertion of CO2 into a metal-hydride bond is observed, resulting in the formation of the expected formate complex, [MoH2(HCOO)(depe)2]+. Further reactivity is triggered at temperature above 100 °C. Complete conversion into two new complexes is indeed observed, resulting from the formal cleavage of a C-O bond of carbon dioxide, [MoH(CO)2(depe)2]+ and [MoO(HCOO)(depe)2]+. Unprecedented in the absence of ligand assistance, such metal hydride reactivity has been comprehensively studied by a combination of experimental and computational means with the aim of elucidating the underlying mechanism that governs this process.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
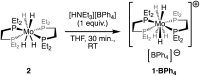


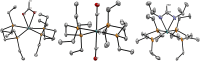
References
LinkOut - more resources
Full Text Sources
Miscellaneous

