Changes to inflammatory markers during 5 years of viral suppression and during viral blips in people with HIV initiating different integrase inhibitor based regimens
- PMID: 39600696
- PMCID: PMC11590120
- DOI: 10.3389/fimmu.2024.1488799
Changes to inflammatory markers during 5 years of viral suppression and during viral blips in people with HIV initiating different integrase inhibitor based regimens
Abstract
Background: Heightened levels of inflammatory markers are linked to increased morbidity/mortality in people with HIV (PWH) and often remain elevated after virologic suppression by antiretroviral therapy (ART). As new combinations of ART become available, an evaluation of their effects on immune activation and inflammation is warranted. Additionally, it remains unknown whether transient increases in viral load ("blips") during ART are associated with increases in inflammation.
Methods: We utilized cryopreserved samples from treatment-naïve PWH enrolled in two Phase 3 clinical trials investigating the efficacy and safety of bictegravir, emtricitabine and tenofovir alafenamide (B/F/TAF) or dolutegravir, abacavir, and lamivudine (DTG/ABC/3TC) or DTG + F/TAF over a 5-year window (GS-US-380-1489/1490). At week 144, participants were offered the option to switch to open label B/F/TAF for an additional 96 weeks. We measured levels of interleukin-6 (IL-6), C-reactive protein (hsCRP), D-dimer, soluble CD14 (sCD14), and tumor necrosis factor-α receptor 1 (TNFR1) from available baseline, week 24, 48, 144, and 240 samples (B/F/TAF, N=123; DTG/ABC/3TC, N=62; DTG+F/TAF, N=58). Additional samples from PWH who experienced a viral blip (n=44, defined as a single HIV-1 RNA >50c/mL) were also analyzed and paired with the most recent available suppressed sample before the blip. Longitudinal biomarker changes were assessed using a constrained mixed effects linear regression model adjusting for covariates.
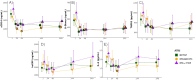
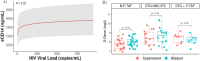
Results: Baseline demographics and selected laboratory characteristics were similar across groups. Levels of D-dimer, sCD14, and TNFR1 decreased significantly from baseline in all treatment arms, with no significant differences between arms at any timepoint. Biomarker levels also remained stable following ART-switch at week 144. No significant changes in hsCRP or IL-6 were observed versus baseline in any arm at any timepoint. A significant association was observed between sCD14 and increasing viral load (p=0.022) in viral blips; D-dimer also increased with blips in the B/F/TAF arm.
Conclusions: Viral suppression was associated with reductions in most inflammatory markers in PWH, with no significant differences among the three ART regimens during the 144-week randomized period. These decreases were sustained after the open label switch to B/F/TAF. Viral blips were associated with increases in monocyte activation (sCD14). Further analysis is needed to confirm these findings and determine the potential impact on clinical outcomes.
Keywords: HIV-1; antiretroviral therapy; inflammation; intermittent viremia; monocyte activation.
Copyright © 2024 Funderburg, Huang, Cohen, Ailstock, Cummings, Lee, Ng, White, Wallin, Downie and McComsey.
Conflict of interest statement
Authors SH, CC, JL, BN, KW, JW, and BD were employed by the company Gilead Sciences Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Psomas C, Younas M, Reynes C, Cezar R, Portales P, Tuaillon E, et al. One of the immune activation profiles observed in HIV-1-infected adults with suppressed viremia is linked to metabolic syndrome: The ACTIVIH study. EBioMedicine. (2016) 8:265–76. doi: 10.1016/j.ebiom.2016.05.008 - DOI - PMC - PubMed
-
- Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. (2014) 210:1248–59. doi: 10.1093/infdis/jiu254 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

