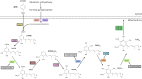
Clinical Features, Biochemistry, Imaging, and Treatment Response in a Single-Center Cohort With Coenzyme Q10 Biosynthesis Disorders
- PMID: 39601013
- PMCID: PMC11595325
- DOI: 10.1212/NXG.0000000000200209
Clinical Features, Biochemistry, Imaging, and Treatment Response in a Single-Center Cohort With Coenzyme Q10 Biosynthesis Disorders
Abstract
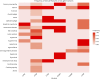
Background and objectives: Disorders of coenzyme Q10 (CoQ10) biosynthesis comprise a group of 11 clinically and genetically heterogeneous rare primary mitochondrial diseases. We sought to delineate clinical, biochemical, and neuroimaging features of these disorders, together with outcomes after oral CoQ10 supplementation and the utility of peripheral blood mononuclear cell (PBMNC) CoQ10 levels in monitoring therapy.
Methods: This was a retrospective cohort study, registered as an audit at a specialist pediatric hospital (Registration Number: 3318) of 14 patients with genetically confirmed CoQ10 biosynthesis deficiency, including 13 previously unreported cases.
Results: We show that oral doses of CoQ10 up to 70 mg/kg/d were needed to ameliorate neurologic features. Additional idebenone was required to control seizures in some cases, and 3 children with neonatal-onset neurologic disease died in early childhood despite receiving high-dose oral CoQ10 from birth. We also demonstrate that early diagnosis and treatment of CoQ10 deficiency with oral supplementation (30 mg/kg/d) can reverse renal manifestations and can completely prevent kidney disease over 10 years of follow-up. PBMNC CoQ10 levels increased after oral CoQ10 supplementation, demonstrating absorption of exogenous CoQ10 into the bloodstream.
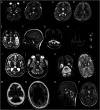
Discussion: An early genome-wide diagnostic approach is needed for expeditious diagnosis of CoQ10 biosynthesis disorder because our study demonstrates that there are no pathognomonic blood, muscle, or imaging biomarkers of these diseases. Our findings indicate that earlier diagnosis and treatment with high-dose CoQ10 is key in halting progression of kidney disease or preventing it altogether. This study uses serial PBMNC CoQ10 levels to monitor therapy. Patients with genetically confirmed CoQ10 biosynthesis disorder should receive high-dose oral CoQ10 as soon as possible after presentation, regardless of genetic cause, to prevent disease progression, but parents of children with neonatal or infantile neurologic presentations should be counseled about the poor prognosis.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
The authors report no relevant disclosures. Go to Neurology.org/NG for full disclosures.
Figures

References
LinkOut - more resources
Full Text Sources
