Relative frequency, characteristics, and disease burden of patients with migraine unsuitable for triptan treatment: A systematic literature review
- PMID: 39601097
- PMCID: PMC11726002
- DOI: 10.1111/head.14854
Relative frequency, characteristics, and disease burden of patients with migraine unsuitable for triptan treatment: A systematic literature review
Abstract
Objective: This review was conducted to systematically identify evidence characterizing patients with migraine who are unsuitable for triptans.
Background: Triptans are not suitable as first-line treatment for all patients with migraine due to contraindications, lack of efficacy, and/or poor tolerability. However, there is debate about the frequency and characteristics of these patients and the burden they experience.
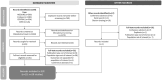
Methods: MEDLINE, Embase, and conference abstracts (2011-2022) were reviewed for evidence on patients with migraine unsuitable for triptans for any reason. Data from publications describing the frequency and characteristics of this group, as well as the clinical, humanistic, or economic burden of disease in this population, were extracted.
Results: Of 1460 records screened, 29 publications met inclusion criteria. Persistence with triptans was low; 51%-66% of patients starting a new triptan did not refill it, and 43%-100% discontinued their initial triptan over 2 years. In one study, 14% of patients with migraine reported prior discontinuation/failure of ≥ 2 triptans due to inadequate efficacy or poor tolerability. Up to 15% of patients with migraine had triptan contraindications, and ≥ 20% of patients receiving triptans had contraindications. In four studies, 10%-44% of patients who tried triptans had insufficient response, although definitions varied. Patients who achieved a sufficient response typically did so with their first triptan; few became responders with additional triptans. Of patients who did not respond to one to two triptans and received another, 45% were dissatisfied with the final triptan. Approximately half of patients who tried two to three triptans had an insufficient response. Greater disability, impact of disease, and depression were reported in triptan discontinuers compared to those with sustained use. Worse quality of life scores and utility values were reported in triptan insufficient versus sufficient responders, as were greater migraine-related costs, work impairment, and health-care resource utilization.
Conclusion: The total population of patients unsuitable for triptans is uncertain, but the literature highlights a large group who cannot or do not persist with triptans, and current evidence suggests a high burden in this population and an unmet need for new therapeutic options. Further research is needed to determine the frequency of unsuitability for triptans more precisely and to assess the associated burden.
Keywords: insufficent response; migraine; treatment; triptan.
© 2024 The Author(s). Headache: The Journal of Head and Face Pain published by Wiley Periodicals LLC on behalf of American Headache Society.
Conflict of interest statement
Figures






References
-
- Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1‐211. - PubMed
-
- Mayans L, Walling A. Acute migraine headache: treatment strategies. Am Fam Physician. 2018;97(4):243‐251. - PubMed
-
- Ailani j, Burch R, Robbins MS, Board of Directors of the American Headache Society . The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021‐1039. - PubMed
-
- Safiri S, Pourfathi H, Eagan A, et al. Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain. 2022;163(2):e293‐e309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

