A Phase I Trial of the Pharmacokinetic Interaction Between Cannabidiol and Tacrolimus
- PMID: 39601108
- PMCID: PMC11835423
- DOI: 10.1002/cpt.3504
A Phase I Trial of the Pharmacokinetic Interaction Between Cannabidiol and Tacrolimus
Abstract
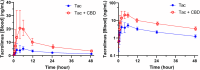
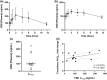
One in six Americans uses cannabidiol-based or cannabis-derived products. Cannabidiol is a substrate of CYP3A, but its role as a potential CYP3A inhibitor remains unclear. We hypothesized that cannabidiol would inhibit CYP3A-mediated metabolism of tacrolimus. This report is an interim analysis of an open-label, three-period, fixed-sequence, crossover study in healthy participants. Participants first received a single dose of tacrolimus 5 mg orally. After washout, participants later received cannabidiol titrated to 5 mg/kg twice daily for 14 days to reach a steady state, followed by a second single dose of tacrolimus 5 mg orally. Tacrolimus concentrations in whole blood were measured by UHPLC-MS/MS method. Pharmacokinetic parameters were calculated by noncompartmental analysis. Twelve participants completed all periods of the study. The maximum concentration (Cmax) of tacrolimus increased 4.2-fold (P < 0.0001) with cannabidiol (40.2 ± 13.5 ng/mL) compared with without cannabidiol (9.85 ± 4.63 ng/mL). The area under the concentration-vs.-time curve (AUC0-∞) increased 3.1-fold (P < 0.0001). No change in half-life (t1/2) was observed. This study demonstrates that cannabidiol increases tacrolimus exposure. Our data suggest the need for dose reduction in tacrolimus and frequent therapeutic dose monitoring in transplant patients taking cannabidiol concomitantly. Whether this observed interaction occurred due to the inhibition of CYP3A4 and/or CYP3A5 in the liver, intestine, or both, or intestinal drug transporters (e.g., p-glycoprotein) during the first-pass elimination remains to be elucidated.
© 2024 The Author(s). Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures

References
-
- OPTN/SRTR 2022 Annual Data Report . Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) <http://srtr.transplant.hrsa.gov/annual_reports/Default.aspx> (2024). Accessed March 1, 2024.
-
- Zuo, X.C. et al. Effects of CYP3A4 and CYP3A5 polymorphisms on tacrolimus pharmacokinetics in Chinese adult renal transplant recipients: a population pharmacokinetic analysis. Pharmacogenet. Genomics 23, 251–261 (2013). - PubMed
-
- Shuker, N. , van Gelder, T. & Hesselink, D.A. Intra‐patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant. Rev. 29, 78–84 (2015). - PubMed
-
- Haufroid, V. , Mourad, M. , Van Kerckhove, V. et al. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics 14, 147–154 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

