A Phase 1b/2a Trial of a Half-life Extended Respiratory Syncytial Virus Neutralizing Antibody, Clesrovimab, in Healthy Preterm and Full-term Infants
- PMID: 39601265
- PMCID: PMC11911779
- DOI: 10.1093/infdis/jiae581
A Phase 1b/2a Trial of a Half-life Extended Respiratory Syncytial Virus Neutralizing Antibody, Clesrovimab, in Healthy Preterm and Full-term Infants
Abstract
Background: Clesrovimab is an investigational monoclonal antibody with an extended half-life targeting site IV of the respiratory syncytial virus (RSV) fusion protein for the prevention of RSV disease in infants.
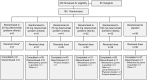
Methods: In this phase 1b/2a, double-blind study, 183 healthy preterm and full-term infants 2 weeks to 8 months of age were randomized 4:1 within 5 panels (preterm 20, 50, 75, or 100 mg; full-term 100 mg) to receive 1 dose of clesrovimab or placebo. The objectives were to evaluate safety, pharmacokinetics, serum neutralizing antibodies (SNA), and antidrug antibodies (ADA). The incidence of RSV-associated end points (medically attended lower respiratory tract infection, hospitalization, and acute respiratory infection) were also evaluated through 150 days postdose.
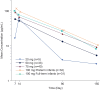
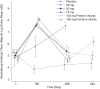
Results: The most common adverse event through day 14 was irritability; no treatment-related serious AEs were reported. Clesrovimab serum concentrations displayed a geometric mean apparent half-life of 44.9 days. Of participants receiving clesrovimab, 51 (36.7%) developed ADA with no apparent impact in pharmacokinetics. SNA titers increased in a dose-dependent manner at day 150. The incidences of RSV-associated end points were lower in infants treated with clesrovimab compared with placebo.
Conclusions: Clesrovimab was generally well tolerated and exhibited an extended half-life compared to typical IgG1 antibodies, supporting its ongoing development in late-stage trials. Clinical Trial Registration. NCT03524118.
Keywords: RSV; clesrovimab; monoclonal antibody.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. R. A. R., B. M. M., X. Z., A. K., B. R., and A. S. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, USA and may hold stock in Merck & Co, Inc, Rahway, NJ, USA. X. C., A. O. A., and A. W. L. were employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, USA at the time of the study and may hold stock in Merck & Co, Inc, Rahway, NJ, USA. K. A. V. is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, USA may hold stock in Merck & Co, Inc, Rahway, NJ, USA and is named on a patent related to MK-1654. J. S. S. reports grants to her institution for serving as a principal investigator for the study. S. A. M. reports payment to his institution for participation in the clinical trial from Merck & Co, Inc, Rahway, NJ, USA; grants to his institution from BMGF, GSK, Pfizer, and Minervax; clinical trial grants to his institution from Novavax, Providence, Gritstone, and ImmunityBio; and participation in a data safety monitoring board for rotavirus vaccine at PATH and HIV monoclonal antibody at CAPRISA. E. A. F. S. reports grants or contracts from Astra Zeneca, Inc, Merck & Co, Inc, Rahway, NJ, USA, Pfizer, Inc, Sanofi Pasteur, Roche Pharmaceuticals, and Johnson and Johnson; consulting fees to his institution from Merck & Co, Inc, Rahway, NJ, USA, Pfizer, Inc, Sanofi Pasteur, Cidara Therapeutics, Adiago Therapeutics, Nuance Pharmaceuticals, Icosavax, Johnson and Johnson, and Sobi, Inc; payments or Honoria for lectures or presentations for Pfizer, Inc, and Astra Zeneca, support for attending meetings or travel from Astra Zeneca, and participation on a data safety monitoring board or advisory board for Abbvie, Inc, GlaxoSmithKline, and The Bill and Melinda Gates Foundation. J. M. N. P. reports grants or contracts from MSD as a principal investigator in MK-1654 clinical trials. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis 2018; 217:1356–64. - PubMed
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140:543–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

