Unraveling the gut-skin axis in atopic dermatitis: exploiting insights for therapeutic strategies
- PMID: 39601281
- PMCID: PMC11610564
- DOI: 10.1080/19490976.2024.2430420
Unraveling the gut-skin axis in atopic dermatitis: exploiting insights for therapeutic strategies
Abstract
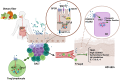
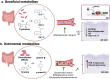
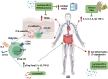
Gut microbiota exert functions of high importance in the intestine. Furthermore, there is increasing evidence for its role in immune regulation and maintenance of homeostasis in many physiological processes taking place in distant tissues. In particular, in this review, we explore the impact of metabolites produced by the gut microbiota on the development of atopic dermatitis (AD). Probiotics and prebiotics balance the microbiota and promote the generation of bacterial metabolites, such as short-chain fatty acids and tryptophan derivates, which promote the regulation of the exacerbated AD immune response through regulatory T cells and IL-10 and TGF-β cytokines. Metabolites also have a direct action on keratinocytes once they reach the bloodstream. Besides, probiotics decrease the levels of metabolites associated with AD onset, such as phenols. Understanding all these crosstalk processes between the gut and the skin reveals a number of possibilities, mainly through the manipulation of the gut microbiome, which may represent therapeutic strategies that can contribute to the standard treatments of AD patients to improve their quality of life.
Keywords: Atopic dermatitis; gut microbiome; gut-skin axis; phenols; prebiotics; probiotics; short-chain fatty acids; tryptophan metabolites.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
