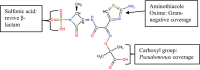
Aztreonam-avibactam: The dynamic duo against multidrug-resistant gram-negative pathogens
- PMID: 39601336
- PMCID: PMC11687205
- DOI: 10.1002/phar.4629
Aztreonam-avibactam: The dynamic duo against multidrug-resistant gram-negative pathogens
Abstract
Antimicrobial resistance poses a significant public health challenge, particularly with the rise of gram-negative hospital-acquired infections resistant to carbapenems. Aztreonam-avibactam (ATM-AVI) is a promising new combination therapy designed to combat multidrug-resistant (MDR) gram-negative bacteria, including those producing metallo-β-lactamases (MBLs). Aztreonam, a monobactam antibiotic, is resistant to hydrolysis by MBLs but can be degraded by other β-lactamases. Avibactam, a novel non-β-lactam β-lactamase inhibitor, effectively neutralizes extended-spectrum β-lactamases (ESBLs) and AmpC β-lactamases, restoring aztreonam's efficacy against resistant pathogens. This review covers the chemistry, mechanisms of action, spectrum of activity, pharmacokinetics, pharmacodynamics, and clinical efficacy of ATM-AVI. ATM-AVI combination has shown efficacy against a wide range of resistant Enterobacterales and other gram-negative bacteria in both in vitro and clinical studies. Pharmacokinetic and pharmacodynamic analyses demonstrate that ATM-AVI maintains effective drug concentrations in the body, with dose adjustments recommended for patients with renal impairment. Clinical trials, including the REVISIT and ASSEMBLE studies, have demonstrated the safety and efficacy of ATM-AVI in treating complicated intra-abdominal infections (cIAI), urinary tract infections (UTIs), and hospital-acquired pneumonia (HAP) caused by MDR gram-negative pathogens. The European Medicines Agency (EMA) has approved ATM-AVI for these indications, and further research is ongoing to optimize dosing regimens and expand its clinical use. This combination represents a critical advancement in the fight against antimicrobial resistance, offering a new therapeutic option for treating severe infections caused by MDR gram-negative, including MBL-producing, bacteria.
Keywords: Enterobacterales; antimicrobial resistance; avibactam; aztreonam; carbapenemase; gram‐negative bacteria; metallo‐β‐lactamase; monobactam.
© 2024 The Author(s). Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy published by Wiley Periodicals LLC on behalf of Pharmacotherapy Publications, Inc.
Conflict of interest statement
Michael J. Rybak has received grant support, consulted, or has participated on a speaker bureau for AbbVie, Innoviva, Melinta, Merck, and Shionogi. All other authors declare no conflicts of interest.
Figures
Similar articles
-
Aztreonam and avibactam combination therapy for metallo-β-lactamase-producing gram-negative bacteria: A Narrative Review.Clin Microbiol Infect. 2025 Jun;31(6):971-978. doi: 10.1016/j.cmi.2024.11.006. Epub 2024 Nov 9. Clin Microbiol Infect. 2025. PMID: 39528085 Review.
-
Aztreonam-avibactam for the treatment of intra-abdominal infections.Expert Opin Pharmacother. 2024 Oct;25(14):1867-1872. doi: 10.1080/14656566.2024.2409950. Epub 2024 Oct 7. Expert Opin Pharmacother. 2024. PMID: 39327993 Review.
-
The Challenge of Treating Infections Caused by Metallo-β-Lactamase-Producing Gram-Negative Bacteria: A Narrative Review.Drugs. 2024 Dec;84(12):1519-1539. doi: 10.1007/s40265-024-02102-8. Epub 2024 Oct 28. Drugs. 2024. PMID: 39467989 Free PMC article. Review.
-
Activity of Aztreonam-avibactam and other β-lactamase inhibitor combinations against Gram-negative bacteria isolated from patients hospitalized with pneumonia in United States medical centers (2020-2022).BMC Pulm Med. 2025 Jan 24;25(1):38. doi: 10.1186/s12890-025-03500-8. BMC Pulm Med. 2025. PMID: 39856702 Free PMC article.
-
Ceftazidime-Avibactam: A Novel Cephalosporin/β-Lactamase Inhibitor Combination for the Treatment of Resistant Gram-negative Organisms.Clin Ther. 2016 Mar;38(3):431-44. doi: 10.1016/j.clinthera.2016.01.018. Epub 2016 Mar 2. Clin Ther. 2016. PMID: 26948862 Review.
Cited by
-
The Changing Landscape of Antibiotic Treatment: Reevaluating Treatment Length in the Age of New Agents.Antibiotics (Basel). 2025 Jul 20;14(7):727. doi: 10.3390/antibiotics14070727. Antibiotics (Basel). 2025. PMID: 40724028 Free PMC article. Review.
-
Whole-genome analysis of NDM-producing Providencia hangzhouensis associated with recurrent bacteraemia with rapid development of aztreonam-avibactam resistance.Emerg Microbes Infect. 2025 Dec;14(1):2539193. doi: 10.1080/22221751.2025.2539193. Epub 2025 Aug 6. Emerg Microbes Infect. 2025. PMID: 40700770 Free PMC article.
References
-
- Centers for Disease Control and Prevention (U.S.) . Antibiotic resistance threats in the United States, 2019. Centers for Disease Control and Prevention (U.S.); 2019. doi:10.15620/cdc:82532 - DOI
-
- COVID‐19: U.S . Impact on antimicrobial resistance, special report 2022. National Center for emerging and zoonotic. Infect Dis. 2022. doi:10.15620/cdc:117915 - DOI
-
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022. World Health Organization; 2022. Licence: CC BY‐NC‐SA 3.0 IGO.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous