Salidroside and exercise performance in healthy active young adults - an exploratory, randomized, double-blind, placebo-controlled study
- PMID: 39601362
- PMCID: PMC11610317
- DOI: 10.1080/15502783.2024.2433744
Salidroside and exercise performance in healthy active young adults - an exploratory, randomized, double-blind, placebo-controlled study
Abstract
Background: Rhodiola rosea extract is purported to improve physical performance and support resilience to stress. Salidroside is considered to be one of the main constituents responsible for the ergogenic actions of R. rosea. However, R. rosea extract contains relatively little salidroside and cultivation of R. rosea is challenging as it is mainly found in high-altitude, cold regions. Additionally, the R. rosea plant is subject to conservation concerns because of its growing popularity. The purpose of this exploratory study was to evaluate the short-term effects of pure, biosynthetic salidroside supplementation on exercise performance, mood state, and markers of inflammation and muscle damage in healthy active young adults.
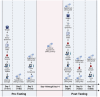
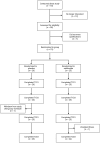
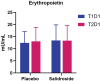
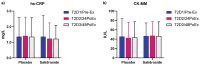
Methods: Fifty participants (30 M, 20F; 21 ± 4 yrs; 173 ± 8 cm; 74 ± 13 kg) were randomly assigned to either salidroside (60 mg/day for 16 days) or placebo supplementation and underwent peak oxygen uptake (VO2 peak), intermittent time-to-exhaustion (TTE), and local muscular endurance assessments, along with mood state evaluations using the Profile of Mood States (POMS). Blood samples were analyzed for erythropoietin, myoglobin, creatine kinase-MM, and C-reactive protein.
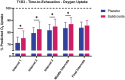
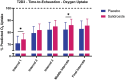
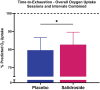
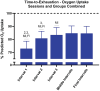
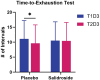
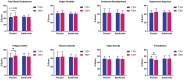
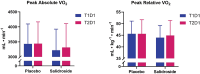
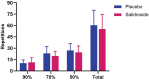
Results: Salidroside supplementation enhanced overall percent predicted oxygen uptake during high-intensity intermittent exercise (p < 0.01). An increase in serum myoglobin was observed 24 hours following exercise in the placebo group (p = 0.02) compared with baseline whereas no statistically significant increase was observed for the salidroside group indicating reduced exercise-induced muscle damage. Placebo group experienced a decrease in number of intervals performed during the TTE test (p = 0.03), and a decrease in friendliness (p < 0.01) and an increase in fatigue-inertia (p < 0.01) as reported by POMS. The salidroside group exhibited stable mood states and maintained performance levels during the time-to-exhaustion test.
Conclusion: Salidroside supplementation may enhance oxygen utilization and mitigate exercise-induced muscle damage and fatigue, warranting further research on its long-term effects and potential as an adaptogen for active individuals.
Keywords: Salidroside; adaptogen; golden root; high-intensity interval exercise; oxygen consumption; rhodiola rosea.
Conflict of interest statement
N.A.S. has a perceived conflict of interest as the recipient of funds used to perform the study. N.A.S. has no financial or commercial interest in the outcome of the study and declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. M.T.S, R.J.C., A.M.M., C.C.W., H.D., M.C.D., and G.M.H. have no commercial or financial interests in the outcome of the study. F.M. and M.S. are employees of Gnosis by Lesaffre, Lesaffre Group. C.R.V., H.C.S., and P.G.K. were employees of DoubleRainbow Biosciences Inc. at the time of study implementation. C.R.V. is an employee of Recombia Biosciences by Lesaffre. J.-K.W. is a member of the Scientific Advisory Board and a shareholder of DoubleRainbow Biosciences, Galixir and Inari Agriculture, which develop biotechnologies related to natural products, drug discovery, and agriculture.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
